Dr Heather Murray
Lecturer / Postdoctoral Researcher
School of Biomedical Sciences and Pharmacy
- Email:heather.murray@newcastle.edu.au
- Phone:0249216934
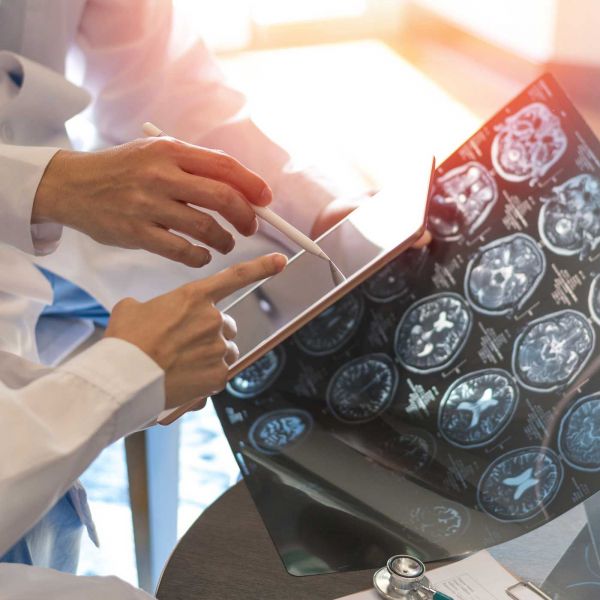
Improving survival outcomes for AML using novel approaches
Acute myeloid leukaemia (AML) is an aggressive and tough-to-cure blood cancer. Through her potentially life-saving work, Dr Heather Murray is developing precision therapies that target the unique biology of AML cells to tackle treatment resistance.

Heather is a postdoctoral cancer researcher at the University of Newcastle, driven by the potential of science to improve treatments for cancer patients.
Originally planning a career in pharmacy, she found her undergraduate degree in biomedical science ignited a passion for research.
“In Biomed, I was exposed to the work of several talented local researchers, and I became interested in the ways that science could be applied to solve biological mysteries and improve treatment outcomes for people who have cancer,” Heather explains.
Her research journey began with summer scholarships and an early focus on melanoma. She later approached Associate Professor Verrills, eager to pursue a PhD project with maximum translational impact.
“I chose to work on leukaemia biology because it was unlike anything I’d done before and had the potential to make a huge difference,” she says.
Heather was awarded her doctorate by the University of Newcastle in 2020. A postdoctoral research position in the team of Associate Professor Verrills followed.
Heather’s PhD study into a novel therapeutic strategy for AML with mutations in the tyrosine kinase 3 (FLT3) gene, which is mutated in approximately one-third of AML cases, has garnered international attention.
Heather is the lead author of research findings from a collaboration between the University of Newcastle, Hunter Medical Research Institute, Hunter Cancer Research Alliance, and the University of Southern Denmark.
In 2021, these findings were published in Leukemia, a prestigious international journal specialising in blood cancer.
Progress in an underexplored area
Each year, approximately 1,100 Australians are diagnosed with acute myeloid leukaemia (AML), a disease that predominantly affects older adults.
AML is one of the most aggressive blood cancers, with a 5-year survival rate of less than 30 per cent. Heather’s research focuses on improving survival outcomes for AML patients by exploring novel approaches to overcome treatment resistance.
“Because AML is relatively rare, it hasn’t received as much research focus as more common cancers,” says Heather.
“The onset of symptoms is very sudden, and the disease progresses rapidly, making treatment selection challenging. However, survival rates have been steadily increasing, and I’m hopeful that further research will continue this trend.”
Targeting a DNA repair protein
“Every individual’s leukaemia is different”, shares Heather. “This reality means that a treatment that works for one person might not be effective for another.”
“Because of this, we need to develop precision therapies tailored to each patient’s cancer. This involves identifying the specific features of AML cells that drive their growth, division and spread.”
“From here, we try and develop tailored therapies targeting these specific features,” she explains.
Analysing cancer cells through methods like proteomics, phosphoproteomics, and genetic mutation studies offers deep insights into cancer biology.
Phosphoproteomics, which examines protein modifications like phosphorylation (a ‘cellular on-off switch’), is particularly useful when combined with other techniques.
In a recent study, the team focused on analysing cells with a KIT gene mutation found in about 5 per cent of acute myeloid leukemia (AML) cases.
Their findings reveal that DNA-dependent protein kinase (DNA-PK) remains continuously active in leukemia cells with the KIT mutation. As a result, they explore the effect of inhibiting both mutant KIT and DNA-PK to halt the growth of these cancerous cells.
By combining standard-of-care drugs with a DNA-PK inhibitor, the treatment significantly reduced leukemia cells while sparing healthy ones.
Heather and her team are optimistic that this strategy, which minimises side effects early in the drug development process, will lead to a well-tolerated therapy.
She hopes her findings will quickly translate into clinical trials to accelerate the discovery of new treatments.
Seeking a clinician’s expertise
The team’s work involves working closely with clinician-researcher Associate Professor Anoop Enjeti, who brings unique insight to our work, making sure they’re always working towards the goal of taking their work into the clinic.
The collaboration with Dr Enjeti is also crucial to the process of testing potential therapies by facilitating the collection of patient cell donations.
“If a patient arrives at the clinic and they consent for some of their samples being used for our research, we can then analyse these cells in the lab and test their response to potential new therapies”, Heather explains.
Importance of research funding
Despite her successes in the field so far, Heather shares that being an emerging researcher comes with the challenge of balancing multiple responsibilities.
These include applying for grants to secure research funding, supervising staff and students, managing research projects, and spending time in the lab conducting experiments.
In a competitive field like this, securing funding as a new researcher can be particularly tough. But it’s so important.
In 2022, Heather received a Cancer Institute NSW fellowship to pursue research into new therapeutic targets for AML.
In 2023, Heather and her team secured a grant to investigate drug resistance in AML, generously funded by Cure Cancer's BarbeCURE®. And her funding was extended in 2024, thanks to the people at Bobbin Head Cruising Club.
“Funding like this enables me to keep working toward more effective precision therapies for AML patients. By investing in early-career researchers, you’re not only driving crucial cancer research forward but also helping to shape the future of scientific discovery.”
Driven by each diagnosis
The groundbreaking work of Heather and her team shines a ray of hope for AML patients in their battle against this formidable cancer.
This potential to help those in need of effective treatment solutions for leukaemia drives her research—every day, she feels it’s real, raw purpose.
“Each time a new sample arrives in the lab, I’m reminded that these cancer cells come from a patient—someone’s parent, sibling, or friend. Someone whose life has been turned upside down by this diagnosis. This motivates me to keep working towards a cure.”
Improving survival outcomes for AML using novel approaches
Heather is a postdoctoral cancer researcher at the University of Newcastle, driven by the potential of science to improve treatments for cancer patients. Originally planning a career in pharmacy, she found her undergraduate degree in biomedical science ignited a passion for research.
Career Summary
Biography
Dr Heather Murray is a Postdoctoral Researcher at The University of Newcastle and the Hunter Medical Research Institute (HMRI). Her research is centred on precision medicine approaches, with a particular emphasis on the aggressive blood cancer, acute myeloid leukaemia (AML).
Dr Murray investigates how each person’s cancer behaves uniquely—almost like a fingerprint—by studying the genes and proteins within cancer cells. Her work aims to understand why certain treatments are effective for some patients but not others. A key focus of her research is uncovering the mechanisms behind treatment resistance in leukaemia, with the goal of improving patient responses and outcomes.
Dr Murray’ s research has attracted several prestigious awards, including the Cancer Institute NSW Early Career Researcher Fellowship and the Ken Mitchelhill Young Investigator Award from the Australasian Proteomics Society. Her findings have been published in leading journals such as Leukemia and Molecular & Cellular Proteomics.
Beyond the laboratory, Dr Murray contributes actively to the scientific community. She has held leadership roles on committees such as the University of Newcastle School of Biomedical Sciences Early Career Researcher Association (2022–2024) and the HMRI Precision Medicine Program Future Leaders Group (2022–present).
Research Interests:
- Predicting Treatment Response: Developing tools to forecast which therapies will be most effective for individual patients before treatment begins.
- Treatment Resistance: Investigating the molecular changes that allow cancer cells to evade therapy, with the aim of extending the effectiveness of treatments.
- Leukaemia Subtypes: Characterising the molecular profiles of different AML subtypes to enable more personalised treatment strategies, using advanced proteomics techniques to identify novel therapeutic targets.
Partnerships:
I collaborate with healthcare professionals, consumers, and industry partners to ensure my research is practical and relevant to patient's needs. Additionally, I've formed collaborations with researchers both local and international to further my research. This includes researchers at the University of Newcastle, UNSW, UniSA, QIMR Berghofer, and SDU.
Featured articles:
ASBMB Today, Seeking leukemia's Achilles heel
https://www.asbmb.org/asbmb-today/science/030823/seeking-leukemias-achilles-heel
9Honey
https://honey.nine.com.au/latest/acute-myeloid-leukaemia-australia-cancer-research/e2f715fb-8ba2-4059-9692-b3d03b530aed
Qualifications
- Doctor of Philosophy in Medical Biochemistry, University of Newcastle
- Bachelor of Biological Science, University of Newcastle
- Bachelor of Biomedical Sciences (Hons), University of Newcastle
- Master of Philosophy, University of Newcastle
Keywords
- Cancer
- DNA repair
- Leukaemia
- Proteomics
Fields of Research
| Code | Description | Percentage |
|---|---|---|
| 320506 | Medical biochemistry - proteins and peptides (incl. medical proteomics) | 60 |
| 321101 | Cancer cell biology | 40 |
Professional Experience
UON Appointment
| Title | Organisation / Department |
|---|---|
| Lecturer / Postdoctoral Researcher | University of Newcastle School of Biomedical Sciences and Pharmacy Australia |
Awards
Award
| Year | Award |
|---|---|
| 2025 |
Cure Cancer Researcher of the Year 2025 - third place Cure Cancer Australia Foundation |
Prize
| Year | Award |
|---|---|
| 2023 |
Leadership Excellence Award Office of the Vice-Chancellor, The University of Newcastle |
Research Award
| Year | Award |
|---|---|
| 2024 |
The Ken Mitchelhill Young Investigator Australasian Proteomics Society |
| 2023 |
The Kellerman Award College of Health, Medicine & Wellbeing - The University of Newcastle |
| 2022 |
Cancer Institute NSW Early Career fellowship Cancer Instititue NSW |
Teaching
| Code | Course | Role | Duration |
|---|---|---|---|
| JMP |
Joint Medical Program School of Biomedical Sciences and Pharmcy, The University of Newcastle |
Lecturer | 1/2/2022 - 31/12/2022 |
| HUBS3302 |
Bioinformatics and Functional Genomics School of Biomedical Sciences and Pharmcy, The University of Newcastle |
Lecturer | 1/7/2022 - 1/1/0001 |
Publications
For publications that are currently unpublished or in-press, details are shown in italics.
Conference (22 outputs)
| Year | Citation | Altmetrics | Link | |||||
|---|---|---|---|---|---|---|---|---|
| 2023 |
Murray HC, Enjeti AK, Samaraweera S, Brzozowski JS, Miller K, D'Andrea RJ, Verrills NM, 'Proteogenomics Coupled with Ex Vivo Profiling for Therapeutic Targeting in AML', BLOOD, 142 (2023)
|
|||||||
| 2023 |
Verrills NM, Murray HC, Brzozowski JS, Panicker N, Miller K, Messina M, Buckley BJ, Kelso MJ, Enjeti AK, 'Preclinical Evaluation of Bisantrene As Single Agent and in Combination with Decitabine for Acute Myeloid Leukemia', BLOOD, 142 (2023)
|
|||||||
| 2021 |
Chen Y, Murray H, AlMazi J, Brozozwski J, Mannan A, Panicker N, Dun MD, Roselli S, Verrills N, 'Proteogenomics identifies oncogenic signalling pathways regulated by the tumour suppressor, PP2A-B55 alpha', ASIA-PACIFIC JOURNAL OF CLINICAL ONCOLOGY, 17, 24-25 (2021)
|
|||||||
| 2015 |
Dun M, Murray H, Al-mazi J, Kahl R, Flanagan H, Smith N, Enjeti A, Larsen M, Verrills N, 'IDENTIFICATION AND SYNERGISTIC TARGETING OF FLT3-ACTIVATED PATHWAYS IN ACUTE MYELOID LEUKAEMIA', ASIA-PACIFIC JOURNAL OF CLINICAL ONCOLOGY, 11, 1-1 (2015) [E3]
|
|||||||
| Show 19 more conferences | ||||||||
Journal article (23 outputs)
| Year | Citation | Altmetrics | Link | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2025 |
Susanto O, Gruber E, Wun CM, Franich RL, Ma X, Sabouri-Thompson Z, Porter ZJ, Murray HC, Cluse LA, Maher B, Brasacchio D, Martin BP, Fraser PJ, Nikolic I, Mir Arnau G, Sandow JJ, Simpson KJ, Verrills NM, Johnstone RW, Thompson PE, Kats LM, Shortt J, 'Discovery and characterisation of VPRBP/DCAF1 kinase inhibitor analogues as microtubular destabilising agents with potent anti-myeloma activity.', Molecular cancer therapeutics (2025) [C1]
|
||||||||||
| 2025 |
Bond DR, Burnard SM, Uddipto K, Hunt KV, Harvey BM, Steffens Reinhardt L, Lawlor-O’Neill C, Roper EA, Humphries S, Murray HC, Mannan A, Dun MD, de Bock CE, Bowden NA, Enjeti AK, Verrills NM, Riveros C, Lê Cao KA, Lee HJ, 'Hypomethylating agents induce epigenetic and transcriptional heterogeneity with implications for acute myeloid leukemia cell self-renewal', Leukemia, 39, 2275-2280 (2025)
|
||||||||||
| 2024 |
Chen Y, Roselli S, Panicker N, Brzozowski JS, Skerrett-Byrne DA, Murray HC, Verrills NM, 'Proteomic and phosphoproteomic characterisation of primary mouse embryonic fibroblasts', PROTEOMICS, 24 (2024) [C1]
|
Open Research Newcastle | |||||||||
| 2024 |
Mulhall JE, Trigg NA, Bernstein IR, Anderson AL, Murray HC, Sipila P, Lord T, Schjenken JE, Nixon B, Skerrett-Byrne DA, 'Immortalized mouse caput epididymal epithelial (mECap18) cell line recapitulates the in-vivo environment', PROTEOMICS, 24 (2024) [C1]
|
Open Research Newcastle | |||||||||
| 2024 |
Duchatel RJ, Jackson ER, Parackal SG, Kiltschewskij D, Findlay IJ, Mannan A, Staudt DE, Thomas BC, Germon ZP, Laternser S, Kearney PS, Jamaluddin MFB, Douglas AM, Beitaki T, McEwen HP, Persson ML, Hocke EA, Jain V, Aksu M, Manning EE, Murray HC, Verrills NM, Sun CX, Daniel P, Vilain RE, Skerrett-Byrne DA, Nixon B, Hua S, de Bock CE, Colino-Sanguino Y, Valdes-Mora F, Tsoli M, Ziegler DS, Cairns MJ, Raabe EH, Vitanza NA, Hulleman E, Phoenix TN, Koschmann C, Alvaro F, Dayas C, Tinkle CL, Wheeler H, Whittle JR, Eisenstat DD, Firestein R, Mueller S, Valvi S, Hansford JR, Ashley DM, Gregory SG, Kilburn LB, Nazarian J, Cain JE, Dun MD, 'PI3K/mTOR is a therapeutically targetable genetic dependency in diffuse intrinsic pontine glioma', JOURNAL OF CLINICAL INVESTIGATION, 134 (2024) [C1]
|
Open Research Newcastle | |||||||||
| 2024 |
Murray HC, Miller K, Dun MD, Verrills NM, 'Pharmaco-phosphoproteomic analysis of cancer-associated KIT mutations D816V and V560G', PROTEOMICS, 24 (2024) [C1]
|
Open Research Newcastle | |||||||||
| 2024 |
Murray HC, Sillar J, Chambers M, Verrills NM, 'Proteogenomic profiling of acute myeloid leukemia to identify therapeutic targets', EXPERT REVIEW OF PROTEOMICS, 21, 515-528 (2024) [C1]
|
||||||||||
| 2024 |
Skerrett-Byrne DA, Stanger SJ, Trigg NA, Anderson AL, Sipila P, Bernstein IR, Lord T, Schjenken JE, Murray HC, Verrills NM, Dun MD, Pang TY, Nixon B, 'Phosphoproteomic analysis of the adaption of epididymal epithelial cells to corticosterone challenge', ANDROLOGY, 12, 1038-1057 (2024) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Germon ZP, Sillar JR, Mannan A, Duchatel RJ, Staudt D, Murray HC, Findlay IJ, Jackson ER, McEwen HP, Douglas AM, McLachlan T, Schjenken JE, Skerrett-Byrne DA, Huang H, Melo-Braga MN, Plank MW, Alvaro F, Chamberlain J, De Iuliis G, Aitken RJ, Nixon B, Wei AH, Enjeti AK, Huang Y, Lock RB, Larsen MR, Lee H, Vaghjiani V, Cain JE, de Bock CE, Verrills NM, Dun MD, 'Blockade of ROS production inhibits oncogenic signaling in acute myeloid leukemia and amplifies response to precision therapies', SCIENCE SIGNALING, 16 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Murray HC, Miller K, Brzozowski JS, Kahl RGS, Smith ND, Humphrey SJ, Dun MD, Verrills NM, 'Synergistic Targeting of DNA-PK and KIT Signaling Pathways in KIT Mutant Acute Myeloid Leukemia', MOLECULAR & CELLULAR PROTEOMICS, 22 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Smyth SP, Nixon B, Anderson AL, Murray HC, Martin JH, MacDougall LA, Robertson SA, Skerrett-Byrne DA, Schjenken JE, 'Elucidation of the protein composition of mouse seminal vesicle fluid', PROTEOMICS, 22 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Staudt DE, Murray HC, Skerrett-Byrne DA, Smith ND, Jamaluddin MFB, Kahl RGS, Duchatel RJ, Germon ZP, McLachlan T, Jackson ER, Findlay IJ, Kearney PS, Mannan A, McEwen HP, Douglas AM, Nixon B, Verrills NM, Dun MD, 'Phospho-heavy-labeled-spiketide FAIMS stepped-CV DDA (pHASED) provides real-time phosphoproteomics data to aid in cancer drug selection', CLINICAL PROTEOMICS, 19 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2021 |
Skerrett-Byrne DA, Bromfield EG, Murray HC, Jamaluddin MFB, Jarnicki AG, Fricker M, Essilfie AT, Jones B, Haw TJ, Hampsey D, Anderson AL, Nixon B, Scott RJ, Wark PAB, Dun MD, Hansbro PM, 'Time-resolved proteomic profiling of cigarette smoke-induced experimental chronic obstructive pulmonary disease', RESPIROLOGY, 26, 960-973 (2021) [C1]
Background and objective: Chronic obstructive pulmonary disease (COPD) is the third leading cause of illness and death worldwide. Current treatments aim to control symp... [more] Background and objective: Chronic obstructive pulmonary disease (COPD) is the third leading cause of illness and death worldwide. Current treatments aim to control symptoms with none able to reverse disease or stop its progression. We explored the major molecular changes in COPD pathogenesis. Methods: We employed quantitative label-based proteomics to map the changes in the lung tissue proteome of cigarette smoke-induced experimental COPD that is induced over 8 weeks and progresses over 12 weeks. Results: Quantification of 7324 proteins enabled the tracking of changes to the proteome. Alterations in protein expression profiles occurred in the induction phase, with 18 and 16 protein changes at 4- and 6-week time points, compared to age-matched controls, respectively. Strikingly, 269 proteins had altered expression after 8 weeks when the hallmark pathological features of human COPD emerge, but this dropped to 27 changes at 12 weeks with disease progression. Differentially expressed proteins were validated using other mouse and human COPD bronchial biopsy samples. Major changes in RNA biosynthesis (heterogeneous nuclear ribonucleoproteins C1/C2 [HNRNPC] and RNA-binding protein Musashi homologue 2 [MSI2]) and modulators of inflammatory responses (S100A1) were notable. Mitochondrial dysfunction and changes in oxidative stress proteins also occurred. Conclusion: We provide a detailed proteomic profile, identifying proteins associated with the pathogenesis and disease progression of COPD establishing a platform to develop effective new treatment strategies.
|
Open Research Newcastle | |||||||||
| 2020 |
Dun MD, Mannan A, Rigby CJ, Butler S, Toop HD, Beck D, Connerty P, Sillar J, Kahl RGS, Duchatel RJ, Germon Z, Faulkner S, Chi M, Skerrett-Byrne D, Murray HC, Flanagan H, Almazi JG, Hondermarck H, Nixon B, De Iuliis G, Chamberlain J, Alvaro F, de Bock CE, Morris JC, Enjeti AK, Verrills NM, 'Shwachman-Bodian-Diamond syndrome (SBDS) protein is a direct inhibitor of protein phosphatase 2A (PP2A) activity and overexpressed in acute myeloid leukaemia', LEUKEMIA, 34, 3393-3397 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2018 |
Staudt D, Murray HC, McLachlan T, Alvaro F, Enjeti AK, Verrills NM, Dun MD, 'Targeting Oncogenic Signaling in Mutant FLT3 Acute Myeloid Leukemia: The Path to Least Resistance', INTERNATIONAL JOURNAL OF MOLECULAR SCIENCES, 19 (2018) [C1]
|
Open Research Newcastle | |||||||||
| 2018 |
Degryse S, de Bock CE, Demeyer S, Govaerts I, Bornschein S, Verbeke D, Jacobs K, Binos S, Skerrett-Byrne DA, Murray HC, Verrills NM, Van Vlierberghe P, Cools J, Dun MD, 'Mutant JAK3 phosphoproteomic profiling predicts synergism between JAK3 inhibitors and MEK/BCL2 inhibitors for the treatment of T-cell acute lymphoblastic leukemia', LEUKEMIA, 32, 788-800 (2018) [C1]
Mutations in the interleukin-7 receptor (IL7R) or the Janus kinase 3 (JAK3) kinase occur frequently in T-cell acute lymphoblastic leukemia (T-ALL) and both are able to ... [more] Mutations in the interleukin-7 receptor (IL7R) or the Janus kinase 3 (JAK3) kinase occur frequently in T-cell acute lymphoblastic leukemia (T-ALL) and both are able to drive cellular transformation and the development of T-ALL in mouse models. However, the signal transduction pathways downstream of JAK3 mutations remain poorly characterized. Here we describe the phosphoproteome downstream of the JAK3(L857Q)/(M511I) activating mutations in transformed Ba/F3 lymphocyte cells. Signaling pathways regulated by JAK3 mutants were assessed following acute inhibition of JAK1/JAK3 using the JAK kinase inhibitors ruxolitinib or tofacitinib. Comprehensive network interrogation using the phosphoproteomic signatures identified significant changes in pathways regulating cell cycle, translation initiation, mitogen-activated protein kinase and phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)/AKT signaling, RNA metabolism, as well as epigenetic and apoptotic processes. Key regulatory proteins within pathways that showed altered phosphorylation following JAK inhibition were targeted using selumetinib and trametinib (MEK), buparlisib (PI3K) and ABT-199 (BCL2), and found to be synergistic in combination with JAK kinase inhibitors in primary T-ALL samples harboring JAK3 mutations. These data provide the first detailed molecular characterization of the downstream signaling pathways regulated by JAK3 mutations and provide further understanding into the oncogenic processes regulated by constitutive kinase activation aiding in the development of improved combinatorial treatment regimens.
|
Open Research Newcastle | |||||||||
| 2017 |
Murray HC, Dun MD, Verrills NM, 'Harnessing the power of proteomics for identification of oncogenic, druggable signalling pathways in cancer', EXPERT OPINION ON DRUG DISCOVERY, 12, 431-447 (2017) [C1]
Introduction: Genomic and transcriptomic profiling of tumours has revolutionised our understanding of cancer. However, the majority of tumours possess multiple mutation... [more] Introduction: Genomic and transcriptomic profiling of tumours has revolutionised our understanding of cancer. However, the majority of tumours possess multiple mutations, and determining which oncogene, or even which pathway, to target is difficult. Proteomics is emerging as a powerful approach to identify the functionally important pathways driving these cancers, and how they can be targeted therapeutically. Areas covered: The authors provide a technical overview of mass spectrometry based approaches for proteomic profiling, and review the current and emerging strategies available for the identification of dysregulated networks, pathways, and drug targets in cancer cells, with a key focus on the ability to profile cancer kinomes. The potential applications of mass spectrometry in the clinic are also highlighted. Expert opinion: The addition of proteomic information to genomic platforms¿'proteogenomics'¿is providing unparalleled insight in cancer cell biology. Application of improved mass spectrometry technology and methodology, in particular the ability to analyse post-translational modifications (the PTMome), is providing a more complete picture of the dysregulated networks in cancer, and uncovering novel therapeutic targets. While the application of proteomics to discovery research will continue to rise, improved workflow standardisation and reproducibility is required before mass spectrometry can enter routine clinical use.
|
Open Research Newcastle | |||||||||
| 2016 |
Murray HC, Maltby VE, Smith DW, Bowden NA, 'Nucleotide excision repair deficiency in melanoma in response to UVA', EXPERIMENTAL HEMATOLOGY & ONCOLOGY, 5 (2016) [C1]
Background: The causative link between UV exposure and melanoma development is well known, however the mechanistic relationship remains incompletely characterised. UVA ... [more] Background: The causative link between UV exposure and melanoma development is well known, however the mechanistic relationship remains incompletely characterised. UVA and UVB components of sunlight are implicated in melanomagenesis; however the majority of studies have focused on the effects of UVB and UVC light. Interestingly, melanoma tumour sequencing has revealed an overrepresentation of mutations signature of unrepaired UV-induced DNA damage. Repair of UVA-induced DNA damage is thought to occur primarily through the Nucleotide Excision Repair (NER) pathway, which recognises and repairs damage either coupled to transcription (Transcription Coupled Repair; TCR), or through global genome scanning (Global Genome Repair; GGR). Current literature suggests NER is deficient in melanoma, however the cause of this remains unknown; and whether reduced NER activity in response to UVA may be involved in melanoma development remains uncharacterised. In this study we aimed to determine if melanoma cells exhibit reduced levels of NER activity in response to UVA. Methods: Melanocyte and melanoma cell lines were UVA-irradiated, and DNA damage levels assessed by immunodetection of Cyclobutane Pyrimidine Dimer (CPD) and (6-4) Photoproduct [(6-4)PP] lesions. Expression of NER pathway components and p53 following UVA treatment was quantified by qPCR and western blot. Results: UVA did not induce detectable induction of (6-4)PP lesions, consistent with previous studies. Repair of CPDs induced by UVA was initiated at 4 h and complete within 48 h in normal melanocytes, whereas repair initiation was delayed to 24 h and >40 % of lesions remained in melanoma cell lines at 48 h. This was coupled with a delayed and reduced induction of GGR component XPC in melanoma cells, independent of p53. Conclusion: These findings support that NER activity is reduced in melanoma cells due to deficient GGR. Further investigation into the role of NER in UVA-induced melanomagenesis is warranted and may have implications for melanoma treatment.
|
Open Research Newcastle | |||||||||
| 2013 |
Bowden NA, Ashton KA, Vilain RE, Avery-Kiejda KA, Davey RJ, Murray HC, Budden T, Braye SG, Zhang XD, Hersey P, Scott RJ, 'Regulators of Global Genome Repair Do Not Respond to DNA Damaging Therapy but Correlate with Survival in Melanoma', PLOS ONE, 8 (2013) [C1]
|
Open Research Newcastle | |||||||||
| Show 20 more journal articles | |||||||||||
Preprint (9 outputs)
| Year | Citation | Altmetrics | Link | |||||
|---|---|---|---|---|---|---|---|---|
| 2025 |
Brzozowski JS, Sahni S, Murray HC, Watt L, Kiltschewskij D, Cairns MJ, Messina M, Tillett D, Kelso MJ, Verrills NM, 'Bisantrene potentiates tyrosine kinase inhibitor activity in clear cell renal cell carcinoma' (2025)
|
|||||||
| 2025 |
Botha V, Murray H, Acharya S, Pringle K, Smith R, Fisher J, 'Placental Iron Utilisation in Fetal Growth Restriction: Alterations in Mitochondrial Heme Synthesis and Iron-Sulfur Cluster Assembly Pathways' (2025)
|
|||||||
| 2025 |
Fisher J, Wang C, Botha V, Acharya S, Murray H, Schjenken J, Pennell C, Smith R, 'The genetic origin of fetal growth restriction and mitochondrial complex I dysregulation' (2025)
|
|||||||
| Show 6 more preprints | ||||||||
Grants and Funding
Summary
| Number of grants | 17 |
|---|---|
| Total funding | $2,905,439 |
Click on a grant title below to expand the full details for that specific grant.
20253 grants / $959,132
Decoding resistance: Leveraging CellenONE to unlock the functional cancer genome at single-cell resolution$599,132
Funding body: Cancer Institute NSW
| Funding body | Cancer Institute NSW |
|---|---|
| Project Team | Prof Nikki Verrills, Dr Danielle Bond, Prof Matt Dun, Dr Anoop Enjeti, Assoc Prof Heather Lee, Dr James Lynam, Dr Heather Murray, Dr Luiza Steffens Reinhardt, Dr Nick Zdenkowski |
| Scheme | Research Equipment Grant |
| Role | Investigator |
| Funding Start | 2025 |
| Funding Finish | 2025 |
| GNo | G2500729 |
| Type Of Funding | C2400 – Aust StateTerritoryLocal – Other |
| Category | 2400 |
| UON | Y |
Predicting response to venetoclax in acute myeloid leukaemia (AML)$260,000
Funding body: Anthony Rothe Memorial Trust
| Funding body | Anthony Rothe Memorial Trust |
|---|---|
| Project Team | Dr Heather Murray, Prof Andrew Wei, Dr Anoop Enjeti, Dr Donia Moujalled, Dr Donia Moujalled, Prof Nikki Verrills, Prof Andrew Wei |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2025 |
| Funding Finish | 2026 |
| GNo | G2501047 |
| Type Of Funding | C3200 – Aust Not-for Profit |
| Category | 3200 |
| UON | Y |
Targeting therapy resistance in acute myeloid leukaemia$100,000
Funding body: Tour De Cure
| Funding body | Tour De Cure |
|---|---|
| Project Team | Dr Heather Murray, Dr Anoop Enjeti, Prof Nikki Verrills |
| Scheme | Early Career Research Grant |
| Role | Lead |
| Funding Start | 2025 |
| Funding Finish | 2025 |
| GNo | G2401317 |
| Type Of Funding | C1700 - Aust Competitive - Other |
| Category | 1700 |
| UON | Y |
20244 grants / $997,986
Mesenchymal Signal Targeting in Myelodysplasia as a pathway to transfusion independence and blood count improvement – the MESSAGE study$871,486
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Dr Anoop Enjeti, Dr Danielle Bond, Dr Belinda Butcher, Dr Robin Gasiorowsko, Dr Devendra Hiwase, Associate Professor Zoe McQuilten, Dr Heather Murray, Prof Andrew Wei, Dr Chun Yew Fong |
| Scheme | MRFF - Early to Mid-Career Researchers Grant |
| Role | Investigator |
| Funding Start | 2024 |
| Funding Finish | 2027 |
| GNo | G2300838 |
| Type Of Funding | C1300 - Aust Competitive - Medical Research Future Fund |
| Category | 1300 |
| UON | Y |
Targeting DNA-PK to overcome resistance to venetoclax in acute myeloid leukaemia (AML)$90,000
Funding body: Cure Cancer Australia Foundation
| Funding body | Cure Cancer Australia Foundation |
|---|---|
| Project Team | Dr Heather Murray |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2024 |
| Funding Finish | 2024 |
| GNo | G2301048 |
| Type Of Funding | C3200 – Aust Not-for Profit |
| Category | 3200 |
| UON | Y |
Decoding paediatric acute myeloid leukaemia (AML): achieving precision in treatment through subtyping$30,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Dr Heather Murray, Prof Nikki Verrills |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2024 |
| Funding Finish | 2024 |
| GNo | G2401761 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
The Mike and Karin Calford Travel Fellowship$6,500
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Dr Heather Murray |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2024 |
| Funding Finish | 2024 |
| GNo | G2301275 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
20232 grants / $104,995
Targeting insulin signalling to overcome resistance to venetoclax in acute myeloid leukaemia (AML)$100,000
Funding body: Cure Cancer Australia Foundation
| Funding body | Cure Cancer Australia Foundation |
|---|---|
| Project Team | Dr Heather Murray, Dr Natasha Anstee, Doctor Natasha Anstee, Prof Matt Dun, Dr Anoop Enjeti, Prof Nikki Verrills, Prof Andrew Wei |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2023 |
| Funding Finish | 2023 |
| GNo | G2200755 |
| Type Of Funding | C1700 - Aust Competitive - Other |
| Category | 1700 |
| UON | Y |
Elucidating markers of response to hypomethylating agents in blood cancers$4,995
Funding body: University of Newcastle
| Funding body | University of Newcastle |
|---|---|
| Project Team | Dr Heather Murray, Dr Jonathan Sillar, Dr Ashwin Unnikrishnan, Prof Nikki Verrills |
| Scheme | Pilot Funding Scheme |
| Role | Lead |
| Funding Start | 2023 |
| Funding Finish | 2023 |
| GNo | G2300457 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
20221 grants / $600,000
Targeting the spliceosome as a novel approach for acute myeloid leukaemia (AML) therapy$600,000
Funding body: Cancer Institute NSW
| Funding body | Cancer Institute NSW |
|---|---|
| Project Team | Dr Heather Murray |
| Scheme | Early Career Fellowship |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2024 |
| GNo | G2100789 |
| Type Of Funding | C2300 – Aust StateTerritoryLocal – Own Purpose |
| Category | 2300 |
| UON | Y |
20212 grants / $126,996
Cracking the Code: The launch of a genomic, epigenetic and proteomic pre-clinical platform to improve the treatment of paediatric leukemias$122,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Matt Dun, Prof Nikki Verrills, Assoc Prof Heather Lee, Dr Janis Chamberlain, Dr Frank Alvaro, Dr Anoop Enjeti, Assoc Prof Kathryn Skelding, Dr Lisa Lincz, Dr Abdul Mannan, Dr Heather Murray, Kristy McCarthy, Elizabeth Heskett, Paola Baeza, Kathleen Irish |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2001337 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
Pilot data for study: Novel therapies for molecular subtypes of acute myeloid leukaemia (AML)$4,996
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Dr Heather Murray |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2100163 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
20192 grants / $10,300
Preclinical research into the potential applications of GDC-0084 in diffuse intrinsic pontine glioma (DIPG)$10,000
Funding body: Kazia Therapeutics Limited
| Funding body | Kazia Therapeutics Limited |
|---|---|
| Project Team | Prof Matt Dun, Associate Professor David Ziegler, Dr Heather Murray, Dr Ryan Duchatel, Dr Frank Alvaro |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2019 |
| Funding Finish | 2019 |
| GNo | G1801161 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Australasian Proteomics Society ECR Travel Award$300
Funding body: Australasian Proteomics Society
| Funding body | Australasian Proteomics Society |
|---|---|
| Scheme | The Human Proteome Organisation Conference 2019 Awards |
| Role | Lead |
| Funding Start | 2019 |
| Funding Finish | 2019 |
| GNo | |
| Type Of Funding | External |
| Category | EXTE |
| UON | N |
20181 grants / $100,000
Proteomic architecture of diffuse pontine intrinsic glioma$100,000
Funding body: McDonald Jones Charitable Foundation
| Funding body | McDonald Jones Charitable Foundation |
|---|---|
| Project Team | Prof Matt Dun, Dr Frank Alvaro, Dr Ryan Duchatel, Dr Heather Murray, Associate Professor David Ziegler |
| Scheme | Postdoctoral fellowship |
| Role | Investigator |
| Funding Start | 2018 |
| Funding Finish | 2020 |
| GNo | G1801130 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
20172 grants / $6,030
HCRA Biomarkers and Targeted Therapies flagship RHD student grant$5,000
Funding body: HCRA Hunter Cancer Research Alliance
| Funding body | HCRA Hunter Cancer Research Alliance |
|---|---|
| Scheme | Research Project |
| Role | Lead |
| Funding Start | 2017 |
| Funding Finish | 2017 |
| GNo | |
| Type Of Funding | Not Known |
| Category | UNKN |
| UON | N |
European Society of Molecular Oncology travel award$1,030
Funding body: ESMO
| Funding body | ESMO |
|---|---|
| Scheme | ESMO Signaling Pathways Symposium 2017 |
| Role | Lead |
| Funding Start | 2017 |
| Funding Finish | 2017 |
| GNo | |
| Type Of Funding | Not Known |
| Category | UNKN |
| UON | N |
Research Supervision
Number of supervisions
Current Supervision
| Commenced | Level of Study | Research Title | Program | Supervisor Type |
|---|---|---|---|---|
| 2025 | PhD | Identifying Biomarkers and Therapeutic Targets for Venetoclax-Resistant Acute Myeloid Leukaemia | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2025 | PhD | Crosstalk Between Epigenetics and Metabolism in Acute Myeloid Leukaemia | PhD (Medical Genetics), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2023 | PhD | Targeting a novel mechanism of therapy resistance in breast cancer | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2023 | PhD | Preclinical testing of a novel strategy for the treatment of Acute Myeloid Leukaemia | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2023 | PhD | Improving Drug Delivery Into The Brain | PhD (Pharmacy), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2022 | PhD | Activation Of A Tumour Suppressor In Triple Negative Breast Cancer | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2022 | PhD | Three-Dimensional Ex Vivo Models of Human Acute Myeloid Leukemia for Predicting Therapeutic Responses and Guiding Precision Medicine | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2020 | PhD | Developing new treatment strategies for therapy resistant breast cancer | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
Past Supervision
| Year | Level of Study | Research Title | Program | Supervisor Type |
|---|---|---|---|---|
| 2024 | PhD | Multiomic Characterisation of Cellular Signalling Regulated by PP2A-B55a in Breast Cancer and Development | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2024 | Honours | Targeting Venetoclax Resistance in Acute Myeloid Leukemia | Biochemistry & Cell Biology, College Health, Medicine and Wellbeing - The University of Newcastle (Australia) | Principal Supervisor |
| 2024 | Honours | Targeting AML oncogenes | Biochemistry & Cell Biology, College of Health, Medicine & Wellbeing - The University of Newcastle | Co-Supervisor |
| 2023 | Honours | The epididymis: a window into natural tumour protection | Biochemistry & Cell Biology, College of Engineering Science and Environment | the University of Newcastle | Australia | Co-Supervisor |
| 2020 | Honours |
Pre-clinical testing of a novel anti-cancer therapy <p>Bachelor of Biomedical Science (Hons)</p> |
Biochemistry & Cell Biology, University of Newcastle | Co-Supervisor |
| 2020 | Honours |
Precision medicine for acute myeloid leukaemia Bachelor of Pharmacy (Hons) |
Biochemistry & Cell Biology, The University of Newcastle | Co-Supervisor |
Research Projects
Verrills Laboratory 2012 -
Grants
A dual approach to activate a tumour suppressor for breast cancer therapy
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Dr Severine Roselli Dayas, Associate Professor Jonathan Morris, Prof Nikki Verrills |
| Scheme | Ideas Grants |
Targeting DNA-PK in acute myeloid leukaemia
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Prof Nikki Verrills, Associate Professor Anoop Enjeti |
| Scheme | Ideas Grants |
Publications
Roberts KG, Smith AM, McDougall FK, Carpenter HC, Horan MP, Neviani P, Powell JA, Thomas D, Guthridge MA, Perrotti D, Sim AT, Ashman LK, Verrills NM, 'Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers', Cancer Research, 70, 5438-5447 (2010) [C1]
Dun MD, Smith AM, Lee EM, Harrison C, Kahl R, Flanagan H, Panicker N, Mashkani B, Don AS, Morris J, Toop H, Lock RB, Powell JA, Thomas D, Guthridge MA, Moore A, Ashman LK, Skelding KA, Enjeti A, Verrills NM, 'Activation of protein phosphatase 2A in FLT3+ acute myeloid leukemia cells enhances the cytotoxicity of FLT3 tyrosine kinase inhibitors.', Oncotarget (2016) [C1]
Murray HC, Dun MD, Verrills NM, 'Harnessing the power of proteomics for identification of oncogenic, druggable signalling pathways in cancer', EXPERT OPINION ON DRUG DISCOVERY, 12, 431-447 (2017) [C1]
Watt LF, Panicker N, Mannan A, Copeland B, Kahl RGS, Dun MD, Young B, Roselli S, Verrills NM, 'Functional importance of PP2A regulatory subunit loss in breast cancer', BREAST CANCER RESEARCH AND TREATMENT, 166, 117-131 (2017) [C1]
Dun MD, Mannan A, Rigby CJ, Butler S, Toop HD, Beck D, Connerty P, Sillar J, Kahl RGS, Duchatel RJ, Germon Z, Faulkner S, Chi M, Skerrett-Byrne D, Murray HC, Flanagan H, Almazi JG, Hondermarck H, Nixon B, De Iuliis G, Chamberlain J, Alvaro F, de Bock CE, Morris JC, Enjeti AK, Verrills NM, 'Shwachman-Bodian-Diamond syndrome (SBDS) protein is a direct inhibitor of protein phosphatase 2A (PP2A) activity and overexpressed in acute myeloid leukaemia', LEUKEMIA, 34, 3393-3397 (2020) [C1]
Panicker N, Coutman M, Lawlor-O'Neill C, Kahl RGS, Roselli S, Verrills NM, 'Ppp2r2aKnockout Mice Reveal That Protein Phosphatase 2A Regulatory Subunit, PP2A-B55 alpha, Is an Essential Regulator of Neuronal and Epidermal Embryonic Development', FRONTIERS IN CELL AND DEVELOPMENTAL BIOLOGY, 8 (2020) [C1]
Murray HC, Enjeti AK, Kahl RGS, Flanagan HM, Sillar J, Skerrett-Byrne DA, Al Mazi JG, Au GG, de Bock CE, Evans K, Smith ND, Anderson A, Nixon B, Lock RB, Larsen MR, Verrills NM, Dun MD, 'Quantitative phosphoproteomics uncovers synergy between DNA-PK and FLT3 inhibitors in acute myeloid leukaemia', LEUKEMIA, 35, 1782-1787 (2021)
Students
| Program | Research Title |
|---|---|
| PhD College of Health, Medicine and Wellbeing |
Multiomic Characterisation of Cellular Signalling Regulated by PP2A-B55a in Breast Cancer and Development |
Collaborators
| Name | Organisation |
|---|---|
| Dr Heather Constance Murray | University of Newcastle |
| Dr Heather Constance Murray | University of Newcastle |
| Doctor Nikita Panicker | University of Newcastle |
| Miss Charley Louise Lawlor-O'Neill | University of Newcastle |
| Dr Lauren Frances Watt | University of Newcastle |
| Dr Lauren Frances Watt | University of Newcastle |
| Miss Kasey Erin Miller | University of Newcastle |
| Mr Joshua Stephen Sidney Brzozowski | University of Newcastle |
| Mr Joshua Stephen Sidney Brzozowski | University of Newcastle |
| Dr Yanfang Chen | University of Newcastle |
| Dr Yanfang Chen | University of Newcastle |
| Dr Severine Roselli Melanie Dayas | University of Newcastle |
| Dr Severine Roselli Melanie Dayas | University of Newcastle |
Edit
News
News • 23 Jun 2025
Researcher honoured for leadership in cancer proteomics
A researcher with the goal to realise individualised medicine for blood cancer has been recognised in Cure Cancer’s prestigious 2025 Researcher of the Year awards, securing third place nationally.
News • 3 Feb 2023
Early-career researchers boosted in mission to beat cancer
On the eve of World Cancer Day on 4 February, four University of Newcastle early career cancer researchers have been awarded $360,000 in grants to support their work investigating blood, gynaecological and colorectal cancers.
News • 15 Dec 2021
Researchers on mission to improve cancer outcomes
Three University of Newcastle researchers have been awarded 2022 Early Career Fellowships by the Cancer Institute NSW. Dr Rebecca Wyse, Dr Yuchen Feng and Dr Heather Murray will each receive a $600,000 scholarship to advance projects designed to improve treatment and outcomes for cancer patients.
News • 2 Nov 2020
New treatment idea discovered for leukaemia
Newcastle researchers have discovered a new way to kill leukaemia cells.
Dr Heather Murray
Position
Lecturer / Postdoctoral Researcher
School of Biomedical Sciences and Pharmacy
College of Health, Medicine and Wellbeing
Contact Details
| heather.murray@newcastle.edu.au | |
| Phone | 0249216934 |
| Link | Research Networks |