Professor Nathan Bartlett
Associate Dean Industry and Engagement
School of Biomedical Sciences and Pharmacy
- Email:nathan.bartlett@newcastle.edu.au
- Phone:0240420171
The fast-evolving landscape of respiratory virus drug development
For a kid who was fascinated by viruses – he found them “pretty cool but a bit scary” – to now be at the forefront of research that could help protect us from a range of respiratory viruses is the realisation of a long-held ambition for viral immunologist Professor Nathan Bartlett.

Nathan found lots of fascinatingly scary viruses during an early-career scholarship with the Australian Centre for Disease Preparedness in Geelong, Victoria, an experience which reinforced his interest in working in virology. From there, he took up a postdoctoral position at Oxford University where his research focused primarily on developing viral vector vaccines using the vaccinia virus, a relative of the virus that causes smallpox. During this time, he learnt a great deal about genetic engineering and cemented his interest in molecular virology and immunology of viruses and vaccines.
But he knew there was something missing. He realised he wanted to be involved with clinical-facing translational research, so took up another postdoctoral position at Imperial College London with a researcher who concentrated primarily on viral exacerbations of asthma and chronic obstructive pulmonary disease (COPD). It became clear to Nathan that the logical next step was to shift his focus to respiratory medicine where, happily, he was able to continue working with those ever-fascinating viruses, particularly the most prevalent human respiratory pathogen, rhinovirus – otherwise known as the common cold virus.
Nathan’s experiences from 13 years in the UK set him on the trajectory to where he is now. He had collaborated with University of Newcastle researchers based at HMRI whilst in the UK, and eventually joined the organisation in 2015 after relocating to Newcastle, somewhere he viewed as more “climactically consistent than Geelong and London” where he could see himself settling and raising a family. Since that time, his translational research has centred on treatments for respiratory viruses, specifically rhinovirus.
“Respiratory viruses cause the clinically most impactful transmissible diseases that challenge societies. There is a huge global burden of respiratory viral infections, with some such as influenza attracting a great deal more research than others such as rhinovirus, even though rhinovirus causes a huge burden of illness,” explains Nathan.
From rhinovirus to coronavirus: a novel control and containment treatment
In his role as head of the Viral Immunology and Respiratory Disease group with HMRI, Nathan has been working to understand the early events that influence the outcome of a respiratory infection. What is the interface between the host’s airway and the virus? How do the airway’s epithelial cells sense and then respond to a virus? How is the innate immune response triggered to control, contain and prevent the viral illness from escalating? What determines whether an infection results in a mild cold, or develops into a more serious lung disease such as an asthma attack or COVID-19? He found that a lot of those early events, mediated by innate immunity, share commonalities across all viral pathogens, which enabled him to translate research that was initially focused on rhinovirus and influenza to other viruses, including coronavirus.
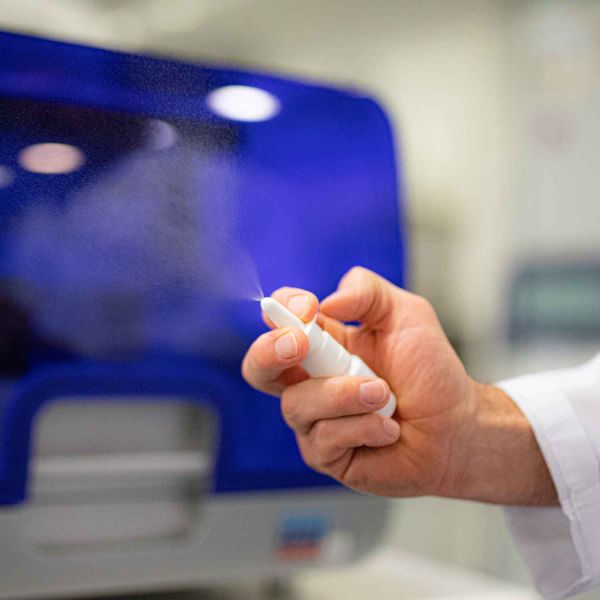
Since 2015, Nathan and his industry partner, Ena Respiratory, have been developing a drug to stimulate the innate immune response in the upper respiratory tract to contain a virus, and thus reduce the risk of it spreading from the upper respiratory tract into the lungs where it can escalate to a severe illness. To date, there are no products that can achieve this outcome. The translational research output is a nasal spray, with Phase 1 clinical trials starting in mid-2021.
Nathan describes his vision for the nasal spray as a protective treatment. “I envisage a day when a person suffering from severe asthma, for example, can walk into a pharmacy and purchase a nasal spray to protect themselves. The product we’re developing will initially be for high-risk individuals including those at high risk of exposure, chronic respiratory disease or immune suppressed. They will administer the nasal spray which will immediately prime for an immune response that facilitates control of the infection in the upper respiratory tract before it has a chance to move through the airways to their lungs.”
When our global village was rapidly engulfed by the coronavirus pandemic early in 2020, Nathan quickly applied the knowledge gained studying the treatment for other respiratory viruses to help show that innate immunity can also be leveraged to tackle coronavirus, offering a potential new treatment approach for COVID-19.
Our community-based virus protective strategies are built around vaccines, but there is a large gap in respiratory virus infection control where vaccines are not available (eg rhinovirus, RSV) or don’t work as effectively as hoped (the elderly and immune suppressed) or are not being used as required (influenza, SARS-CoV-2). This is where the nasal spray will enter the market. It’s important to note that the nasal spray is not intended to take the place of a vaccine. Rather, it will help control and contain a virus, and prevent at-risk groups such as the immunosuppressed, elderly, unvaccinated or those who have not responded well to vaccination from becoming severely ill.
“The aim of the treatment is to keep respiratory viruses from travelling from the nose and throat and entering the lungs where, as we know, it can cause severe illness and death,” Nathan explains.
The real-world application of research: a gratifying experience
After 15 years of researching respiratory viruses, Nathan is proud to say that he has played a significant role in the development of a treatment targeting the upper airways which will prevent these viruses from generating a more serious disease as soon as it causes infection. He is quick to point out that, like all research endeavours, he did not achieve this alone.
He is grateful to the Hunter Medical Research Institute and Asthma Australia who provided the seed funding needed to develop the virus infection models that enabled him to work with Ena Respiratory.
Nathan believes that COVID-19 is going to change the landscape of respiratory virus drug development moving forward. Treatments are now a major global focus, and it’s imperative that research and investment in that area continue to improve.
“I have no doubt that there will be a lot of other medications developed on the back of the urgency around COVID-19. The offshoot of that is that we will soon see better treatments for other viral diseases including RSV bronchiolitis, influenza, and asthma and COPD exacerbations. Playing a role in that has been a gratifying experience.”
The fast-evolving landscape of respiratory virus drug development
For a kid who was fascinated by viruses – he found them “pretty cool but a bit scary” – to now be at the forefront of research that could help protect us from a range of respiratory viruses is the realisation of a long-held ambition for viral immunologist Associate Professor Nathan Bartlett.
Career Summary
Biography
Nathan Bartlett is Professor and head of the Viral Immunology and Respiratory Disease group and is based at the Hunter Medical Research Institute. He also retains an honorary academic appointment at Imperial College London, UK to continue collaborative research.
Following the award of PhD, Dr Bartlett undertook a 5 year Postdoctoral research position, first at the Sir William Dunn School of Pathology, University of Oxford, then in the Department of Virology, Imperial College London. Dr Bartlett then undertook a second Postdoctoral position in the Department of Respiratory Medicine within the National Heart and Lung Institute (NHLI), also at Imperial.
After joining the NHLI, Dr Bartlett continued to build on his virology training leading to Bartlett et al, Nature Medicine 2008, the world's first mouse rhinovirus infection model. He has investigated the interaction of respiratory viral infection with type-2 immunity to uncover pathogenic mechanisms in asthma exacerbations. He was a co-applicant on several successful project and program grants and since his Lecturer appointment at Imperial in 2011 was successful as the Lead Investigator in achieving an MRC project grant to study the role of IL-25 in asthma exacerbations, the research from which was published as the featured cover story in Science Translational Medicine (October 2014). Dr Bartlett has contributed to several scholarly books including the Rhinovirus chapter for the Encyclopaedia of Virology (Elsevier) and ,edited Rhinovirus Infections: Rethinking Impact on Human Health and Disease (Elsevier). Dr Bartlett is considered a world expert on in vivo and human airway epithelial models of rhinovirus infection and consults for a number companies that are bringing novel therapies for respiratory infection and inflammatory airways diseases to the clinic. He is also an Associate Editor for the American Journal of Physiology - Lung, Cellular and Molecular Physiology (AJP-LUNG) and a member of the European Respiratory Society College of Experts.
In 2015 Dr Bartlett accepted an academic appointment at the University of Newcastle where he has received funding from HMRI, Asthma Australia, NH&MRC and multiple Industry Partners and associated Government grants. He work has led to the publishing of multiple patents related to treatments currently in development for respiratory virus infections and exacerbations of chronic respiratory diseases.
He is now exploring novel therapies and treatments to fight COVID-19, a novel coronavirus which was declared a pandemic in March 2020. His team is exploring a range of anti-viral options for this virus, partnering with other researchers to explore different methods to fight the virus. For example Dr Bartlett is working with Professor Hubert Hondermarck to assess the safety and efficacy of a range of cancer drugs which have the potential to be repurposed.
Dr Bartlett continues to work with ENA Respiratory (Brandon BioCatalyst-funded company) who have developed an innate immune stimulant that protects the lungs against respiratory virus infections. Boosting lung innate immunity bridges the gap between vaccine mediated long term protection and anti-viral drug to treat active infection. He is now using coronavirus infections models to determine the efficacy of this approach.
He is also helping local industries that are focusing their business to produce disinfectants, sanitizers and virus inactivating materials that will become part of everyday life as we adapt to coexist with new respiratory viruses such as SARS-CoV-2.
Qualifications
- PhD, Deakin University
- Bachelor of Science (Honours), Deakin University
Keywords
- Asthma
- Epithelium
- Respiratory virus
- Rhinovirus
- Type 2 immunity
- allergy
- anti-viral immunity
- innate immunity
Fields of Research
| Code | Description | Percentage |
|---|---|---|
| 310706 | Virology | 40 |
| 320407 | Innate immunity | 40 |
| 321299 | Ophthalmology and optometry not elsewhere classified | 20 |
Professional Experience
UON Appointment
| Title | Organisation / Department |
|---|---|
| Associate Dean Industry and Engagement | University of Newcastle School of Biomedical Sciences and Pharmacy Australia |
Academic appointment
| Dates | Title | Organisation / Department |
|---|---|---|
| 20/3/2011 - | Lecturer | Imperial College London United Kingdom |
Publications
For publications that are currently unpublished or in-press, details are shown in italics.
Book (1 outputs)
| Year | Citation | Altmetrics | Link | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 |
, 'Rhinovirus infections: Rethinking the impact on human health and disease', 1-314 (2019)
|
||||||||||
Chapter (11 outputs)
| Year | Citation | Altmetrics | Link | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 |
Esneau C, Bartlett N, Bochkov YA, 'Chapter 1 Rhinovirus structure, replication, and classification', 1-23 (2019)
|
||||||||||
| 2019 |
Girkin J, Maltby S, Singanayagam A, Bartlett N, Malia P, 'In vivo experimental models of infection and disease', 195-238 (2019) [B1]
|
Open Research Newcastle | |||||||||
| 2019 |
Girkin J, Maltby S, Singanayagam A, Bartlett N, Mallia P, 'Chapter 8 In vivo experimental models of infection and disease', 195-238 (2019)
|
||||||||||
| 2019 |
Bartlett N, Esneau C, Bochkov Y, 'Rhinovirus structure, replication, and classification', 1-23 (2019) [B1]
|
Open Research Newcastle | |||||||||
| 2019 |
Reid AT, Sutanto EN, Chander-Veerati P, Looi K, Li NF, Iosifidis T, Loo SL, Garratt LW, Kicic A, 'Ground zero-the airway epithelium', 61-98 (2019) [B1]
|
Open Research Newcastle | |||||||||
| 2019 |
Wark P, Williams T, Pathinayake P, 'The interplay of the host, virus, and the environment', 169-194 (2019) [B1]
|
Open Research Newcastle | |||||||||
| 2019 |
McLean G, Girkin J, Solari R, 'Emerging therapeutic approaches', 239-263 (2019) [B1]
|
Open Research Newcastle | |||||||||
| 2015 |
Bartlett NW, Singanayagam A, Johnston SL, 'Mouse models of rhinovirus infection and airways disease', 1221, 181-188 (2015) [B1]
|
Open Research Newcastle | |||||||||
| 2014 |
Bartlett NW, Johnston SL, 'Rhinoviruses' (2014)
© 2014 Elsevier Inc. All rights reserved.Human rhinovirus (HRV) infections are the most frequent cause of the common cold, the most common illness affecting mankind wit... [more] © 2014 Elsevier Inc. All rights reserved.Human rhinovirus (HRV) infections are the most frequent cause of the common cold, the most common illness affecting mankind with cases documented as far back in human history as the ancient Egyptians. Morbidity associated with the illness is a major cause of lost productivity through absenteeism from school or work. More recently, rhinoviruses have been shown to play a significant role in precipitating severe respiratory disease syndromes such as acute exacerbations of asthma. Rhinoviruses belong to the family Picornaviridae. Initially 102 HRV serotypes were identified. Subsequent molecular-based genetic identification has extended the number to over 160 viruses which are divided into 3 species (HRV-A, -B and -C). The immense antigenic variation arising from such a large number of virus types has thwarted efforts to date to develop an anti-HRV vaccine. Viral replication occurs in the cytoplasm and the virions are small non-enveloped icosahedral particles which contain a single-stranded positive-sense RNA genome. The single polyprotein encoded by the RNA genome is cleaved by viral proteases to yield mature proteins. The majority of HRV serotypes require intercellular adhesion molecule 1 for cell binding and entry. Rhinoviruses exhibit a narrow host range infecting only humans and some primates although recently mouse infection models have been developed. Sensitive molecular assays such as polymerase chain reaction (PCR) have significantly increased the ability to detect HRVs direcetly in infected material. Transmitted by aerosol the virus enters via the nose and replicates in the nasal epithelium, inducing production of pro-inflammatory cytokines that contribute to cold symptoms with involvement of lower respiratory tract in susceptible individuals.
|
||||||||||
| Show 8 more chapters | |||||||||||
Conference (48 outputs)
| Year | Citation | Altmetrics | Link | |||||
|---|---|---|---|---|---|---|---|---|
| 2025 |
Berthon B, Williams E, Williams L, Budden K, Hiles S, Bartlett N, Wood L, 'Oral lactoferrin enhances ex vivo immune response to respiratory virus infection, an RCT in older adults', TSANZ Abstract, 55, 4-193 (2025)
|
|||||||
| 2024 |
Bartlett NW, Bryant NE, Jackson CL, White D, Blaiss M, Girkin JLN, 'Blocking IL-25 Inhibits Pulmonary Neutrophilic Inflammation During Rhinovirus Exacerbation of Allergic Airways Disease', AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE, 209 (2024)
|
|||||||
| 2023 |
Williams L, Berthon B, Bartlett N, Wark P, Wood L, 'Bacterial metabolites of dietary fibre fermentation, propionate and butyrate, reduce type 2 cytokine responses by peripheral blood mononuclear cells from subjects with asthma', PROCEEDINGS OF THE NUTRITION SOCIETY, 82 (2023)
|
|||||||
| 2021 |
Ngan FL, Reid A, Nichol K, Grainge C, Wark P, Knight D, Bartlett N, 'Dysregulated actin cytoskeleton associated with barrier dysfunction in asthma', FASEB JOURNAL, 35 (2021)
|
|||||||
| 2014 |
Collison A, Hatchwell L, Girkin J, Parsons K, Li J, Zhang J, Phipps S, Knight D, Bartlett NW, Johnston SL, Foster PS, Wark PAB, Mattes J, 'Late-breaking abstract: IL-5-induced airways eosinophilia as a negative regulator of TLR7 expression may impair the interferon response to rhinovirus in allergic airways', EUROPEAN RESPIRATORY JOURNAL, 44 (2014)
|
|||||||
| 2012 |
Collison AM, Hatchwell LM, Siqueira AP, Bartlett NW, Johnston SL, Foster PS, Mattes J, 'Antagonism of microRNA-122 is comparible to azithromycin treatment in a mouse model of rhinovirus-induced exacerbation of allergic airways disease', Respirology, 17(S1) (2012) [E3]
|
|||||||
| 2012 |
Hatchwell LM, Collison AM, Siqueira AP, Bartlett NW, Johnston SL, Foster PS, Mattes J, 'Toll-like receptor 7 mediates anti-viral responses to rhinovirus while suppressing exacerbation of asthma', Respirology, 17(S1) (2012) [E3]
|
|||||||
| 2012 |
Hatchwell LM, Collison AM, Siqueira AP, Bartlett NW, Johnston SL, Foster PS, Mattes J, 'TRAIL regulates inflammatory responses to rhinovirus and rhinovirus-induced exacerbation of asthma', Respirology, 17(S1) (2012) [E3]
|
|||||||
| Show 45 more conferences | ||||||||
Journal article (104 outputs)
| Year | Citation | Altmetrics | Link | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2026 |
Berthon BS, Williams EJ, Williams LM, Budden KF, Hiles SA, Bartlett NW, Wood LG, 'Oral lactoferrin reduces systemic inflammation, enhances anti-viral responses and modulates immune cell profiles: an RCT in healthy, older adults', British Journal of Nutrition (2026)
|
||||||||||
| 2025 |
Adams TJ, Schuliga M, Pearce N, Bartlett NW, Liang M, 'Targeting respiratory virus-induced reactive oxygen species in airways diseases', European Respiratory Review, 34 (2025) [C1]
|
||||||||||
| 2025 |
Esneau C, Boettiger D, Leask S, Bryant NE, Niessen N, McVernon J, Marcato A, Carlson S, Browne S, Thomas R, Holmes EC, Hajkowicz K, Tilbrook L, Moon N, Dalton C, Bartlett NW, Davis JS, 'The Pandemic REspiratory Virus Epidemiological SurveillaNce Trial - A self-swab surveillance system for respiratory viruses nested within FluTracking.', The Journal of infectious diseases (2025) [C1]
|
||||||||||
| 2025 |
Berthon BS, Williams LM, McLoughlin RF, Nichol KS, Negewo NA, Thompson CA, Williams EJ, Bartlett NW, Wark PAB, Wood LG, 'Pilot investigation of an oligosaccharide blend in adults with asthma – a double-blind, randomised, placebo-controlled, 4-arm crossover trial', Respiratory Medicine, 250 (2025) [C1]
|
||||||||||
| 2025 |
Esneau C, Bryant NE, Johnston SL, Bartlett NW, 'Opportunities for rhinovirus-targeted RNA therapeutics: A narrative review', CMI Communications, 2, 105081-105081 (2025) [C1]
|
||||||||||
| 2024 |
Akerman A, Fichter C, Milogiannakis V, Esneau C, Silva MR, Ison T, Lopez JA, Naing Z, Caguicla J, Amatayakul-Chantler S, Roth N, Manni S, Hauser T, Barnes T, Boss T, Condylios A, Yeang M, Sato K, Bartlett NN, Darley D, Matthews G, Stark DJ, Promsri S, Rawlinson WD, Murrell B, Kelleher AD, Dwyer D, Sintchenko V, Kok J, Ellis S, Marris K, Knight E, Hoad VC, Irving DO, Gosbell I, Brilot F, Wood J, Aggarwal A, Turville SG, 'Cross-sectional and longitudinal genotype to phenotype surveillance of SARS-CoV-2 variants over the fi rst four years of the COVID-19 pandemic', EBIOMEDICINE, 110 (2024) [C1]
Background: Continued phenotyping and ongoing molecular epidemiology are important in current and future monitoring of emerging SARS-CoV-2 lineages. Herein we developed... [more] Background: Continued phenotyping and ongoing molecular epidemiology are important in current and future monitoring of emerging SARS-CoV-2 lineages. Herein we developed pragmatic strategies to track the emergence, spread and phenotype of SARS-CoV-2 variants in Australia in an era of decreasing diagnostic PCR testing and focused cohort-based studies. This was aligned to longitudinal studies that span 4 years of the COVID-19 pandemic. Methods: Throughout 2023, we partnered with diagnostic pathology providers and pathogen genomics teams to identify relevant emerging or circulating variants in the New South Wales (NSW) community. We monitored emerging variants through viral culture, growth algorithms, neutralisation responses and changing entry requirements defined by ACE2 and TMPRSS2 receptor use. To frame this in the context of the pandemic stage, we continued to longitudinally track neutralisation responses at the population level using pooled Intravenous Immunoglobulins (IVIG) derived from in excess of 700,000 donations. Findings: In antibodies derived from recent individual donations and thousands of donations pooled in IVIGs, we observed continued neutralisation across prior and emerging variants with EG.5.1, HV.1, XCT and JN.1 ranked as the most evasive SARS-CoV-2 variants. Changes in the type I antibody site at Spike positions 452, 455 and 456 were associated with lowered neutralisation responses in XBB lineages. In longitudinal tracking of population immunity spanning three years, we observed continued maturation of neutralisation breadth to all SARS-CoV-2 variants over time. Whilst neutralisation responses initially displayed high levels of imprinting towards Ancestral and early pre-Omicron lineages, this was slowly countered by increased cross reactive breadth to all variants. We predicted JN.1 to have a marked transmission advantage in late 2023 and this eventuated globally at the start of 2024. We could not attribute this advantage to neutralisation resistance but rather propose that this growth advantage arises from the preferential utilisation of ACE2 pools that cannot engage TMPRSS2 at its Collectrin-Like Domain (CLD). Interpretation: The emergence of many SARS-CoV-2 lineages documented at the end of 2023 was found to be initially associated with lowered neutralisation responses. This continued to be countered by the gradual maturation of cross-reactive neutralisation responses over time. The later appearance and dominance of the divergent JN.1 lineage cannot be attributed to a lack of neutralisation responses alone, and our data supports that its dominance is a culmination of both lowered neutralisation and changes in ACE2/TMPRSS2 entry preferences. Funding: This work was primarily supported by Australian Medical Foundation research grants MRF2005760 (ST, GM & WDR), MRF2001684 (ADK and ST) and Medical Research Future Fund Antiviral Development Call grant (WDR), Medical Research Future Fund COVID-19 grant (MRFF2001684, ADK & SGT) and the New South Wales Health COVID-19 Research Grants Round 2 (SGT).
|
||||||||||
| 2024 |
Adair A, Tan LL, Feng J, Girkin J, Bryant N, Wang M, Mordant F, Chan L-J, Bartlett NW, Subbarao K, Pymm P, Tham W-H, 'Human coronavirus OC43 nanobody neutralizes virus and protects mice from infection', JOURNAL OF VIROLOGY, 98 (2024) [C1]
|
Open Research Newcastle | |||||||||
| 2024 |
Duque-Sanchez L, Pasic PJ, Esneau C, Batra V, Tjandaputra G, Tan T, Bartlett N, Thissen H, 'Synergistic Polymer Coatings with Antibacterial and Antiviral Properties for Healthcare Applications', ACS OMEGA, 9, 32662-32673 (2024) [C1]
The role of frequently touched surfaces in the transmission of infectious diseases is well-documented, and the urgent need for effective surface technologies with antip... [more] The role of frequently touched surfaces in the transmission of infectious diseases is well-documented, and the urgent need for effective surface technologies with antipathogen activity has been highlighted by the recent global pandemic and rise in antimicrobial resistance. Here, we have explored combinations of up to 3 different classes of compounds within a polymeric matrix to enable the fabrication of coatings with broad-spectrum activity. Compounds were either based on metals or metal oxides, namely, copper, silver, and copper oxide, essential oils, namely, cinnamaldehyde, tea tree oil, and carvacrol oil, or cationic polymers, namely, poly(¿-lysine) and poly(hexamethylene biguanide). These compounds were mixed into a polymer matrix, coated, and dried to yield durable coatings. Coatings containing up to 7.5% (w/w) of the compounds were assessed in the zone of inhibition and biofilm assays using Staphylococcus aureus and Pseudomonas aeruginosa, as well as infectivity assays using human coronavirus OC43. Our data demonstrate that a selected combination of additives was able to provide a 5-log reduction in the colony-forming units of both bacteria and a 4-log reduction in viral infectivity. This simple but highly effective technology is expected to find applications in environments such as hospitals, aged care facilities, or public transport.
|
||||||||||
| 2023 |
Blackburn JB, Li NF, Bartlett NW, Richmond BW, 'An update in club cell biology and its potential relevance to chronic obstructive pulmonary disease', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 324, L652-L665 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Girkin JLN, Bryant NE, Loo S-L, Hsu A, Kanwal A, Williams TC, Maltby S, Turville SG, Wark PAB, Bartlett NW, 'Upper Respiratory Tract OC43 Infection Model for Investigating Airway Immune-Modifying Therapies', AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY, 69, 614-622 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Veerati PC, Reid AT, Nichol KS, Wark PAB, Knight DA, Bartlett NW, Grainge CL, 'Mechanical forces suppress antiviral innate immune responses from asthmatic airway epithelial cells following rhinovirus infection', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 325, L206-L214 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Antunes KH, Singanayagam A, Williams L, Faiez TS, Farias A, Jackson MM, Faizi FK, Aniscenko J, Kebadze T, Veerati PC, Wood L, Bartlett NW, de Souza APD, Johnston SL, 'Airway-delivered short-chain fatty acid acetate boosts antiviral immunity during rhinovirus infection', JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY, 151, 447-+ (2023) [C1]
|
||||||||||
| 2023 |
Dy ABC, Girkin J, Marrocco A, Collison A, Mwase C, O'Sullivan MJ, Phung T-KN, Mattes J, Koziol-White C, Gern JE, Bochkov YA, Bartlett NW, Park J-A, 'Rhinovirus infection induces secretion of endothelin-1 from airway epithelial cells in both in vitro and in vivo models', RESPIRATORY RESEARCH, 24 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Malik B, Bartlett NW, Upham JW, Nichol KS, Harrington J, Wark PAB, 'Severe asthma ILC2s demonstrate enhanced proliferation that is modified by biologics', RESPIROLOGY, 28, 758-766 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2023 |
Akerman A, Milogiannakis V, Jean T, Esneau C, Silva MR, Ison T, Fichter C, Lopez JA, Chandra D, Naing Z, Caguicla J, Li D, Walker G, Amatayakul-Chantler S, Roth N, Manni S, Hauser T, Barnes T, Condylios A, Yeang M, Wong M, Foster CSP, Sato K, Lee S, Song Y, Mao L, Sigmund A, Phu A, Vande More AM, Hunt S, Douglas M, Caterson I, Britton W, Sandgren K, Bull R, Lloyd A, Triccas J, Tangye S, Bartlett NW, Darley D, Matthews G, Stark DJ, Petoumenos K, Rawlinson WD, Murrell B, Brilot F, Cunningham AL, Kelleher AD, Aggarwal A, Turville SG, 'Emergence and antibody evasion of BQ, BA.2.75 and SARS- CoV-2 recombinant sub-lineages in the face of maturing antibody breadth at the population level', EBIOMEDICINE, 90 (2023) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Williams TC, Loo S-L, Nichol KS, Reid AT, Veerati PC, Esneau C, Wark PAB, Grainge CL, Knight DA, Vincent T, Jackson CL, Alton K, Shimkets RA, Girkin JL, Bartlett NW, 'IL-25 blockade augments antiviral immunity during respiratory virus infection', COMMUNICATIONS BIOLOGY, 5 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Singanayagam A, Footitt J, Marczynski M, Radicioni G, Cross MT, Finney LJ, Trujillo-Torralbo M-B, Calderazzo M, Zhu J, Aniscenko J, Clarke TB, Molyneaux PL, Bartlett NW, Moffatt MF, Cookson WO, Wedzicha J, Evans CM, Boucher RC, Kesimer M, Lieleg O, Mallia P, Johnston SL, 'Airway mucins promote immunopathology in virus-exacerbated chronic obstructive pulmonary disease', JOURNAL OF CLINICAL INVESTIGATION, 132 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Girkin JLN, Maltby S, Bartlett NW, 'Toll-like receptor-agonist-based therapies for respiratory viral diseases: thinking outside the cell', EUROPEAN RESPIRATORY REVIEW, 31 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Veerati PC, Nichol KS, Read JM, Bartlett NW, Wark PAB, Knight DA, Grainge CL, Reid AT, 'Conditionally reprogrammed asthmatic bronchial epithelial cells express lower FOXJ1 at terminal differentiation and lower IFNs following RV-A1 infection', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 323, L495-L502 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Aggarwal A, Stella AO, Walker G, Akerman A, Esneau C, Milogiannakis V, Burnett DL, McAllery S, Silva MR, Lu Y, Foster CSP, Brilot F, Pillay A, Van Hal S, Mathivanan V, Fichter C, Kindinger A, Hoppe AC, Munier ML, Amatayakul-Chantler S, Roth N, Coppola G, Symonds GP, Schofield P, Jackson J, Lenthall H, Henry JY, Mazigi O, Jack H-M, Davenport MP, Darley DR, Matthews G, Khoury DS, Cromer D, Goodnow CC, Christ D, Robosa R, Starck DJ, Bartlett NW, Rawlinson WD, Kelleher AD, Turville SG, 'Platform for isolation and characterization of SARS-CoV-2 variants enables rapid characterization of Omicron in Australia', NATURE MICROBIOLOGY, 7, 896-908 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Esneau C, Duff AC, Bartlett NW, 'Understanding Rhinovirus Circulation and Impact on Illness', VIRUSES-BASEL, 14 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
Kan S, Grainge C, Nichol K, Reid A, Knight D, Sun Y, Bartlett N, Liang M, 'TLR7 agonist loaded airway epithelial targeting nanoparticles stimulate innate immunity and suppress viral replication in human bronchial epithelial cells', INTERNATIONAL JOURNAL OF PHARMACEUTICS, 617 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2022 |
George PM, Reed A, Desai SR, Devaraj A, Faiez TS, Laverty S, Kanwal A, Esneau C, Liu MKC, Kamal F, Man WD-C, Kaul S, Singh S, Lamb G, Faizi FK, Schuliga M, Read J, Burgoyne T, Pinto AL, Micallef J, Bauwens E, Candiracci J, Bougoussa M, Herzog M, Raman L, Ahmetaj-Shala B, Turville S, Aggarwal A, Farne HA, Dalla Pria A, Aswani AD, Patella F, Borek WE, Mitchell JA, Bartlett NW, Dokal A, Xu X-N, Kelleher P, Shah A, Singanayagam A, 'A persistent neutrophil-associated immune signature characterizes post-COVID-19 pulmonary sequelae', SCIENCE TRANSLATIONAL MEDICINE, 14 (2022) [C1]
|
Open Research Newcastle | |||||||||
| 2021 |
Contoli M, Papi A, Tomassetti L, Rizzo P, Dalla Sega FV, Fortini F, Torsani F, Morandi L, Ronzoni L, Zucchetti O, Pavasini R, Fogagnolo A, Volta CA, Bartlett NW, Johnston SL, Spadaro S, Campo G, 'Blood Interferon-alpha Levels and Severity, Outcomes, and Inflammatory Profiles in Hospitalized COVID-19 Patients', FRONTIERS IN IMMUNOLOGY, 12 (2021) [C1]
Background: Deficient interferon responses have been proposed as one of the relevant mechanisms prompting severe manifestations of COVID-19. Objective: To evaluate the ... [more] Background: Deficient interferon responses have been proposed as one of the relevant mechanisms prompting severe manifestations of COVID-19. Objective: To evaluate the interferon (IFN)-a levels in a cohort of COVID-19 patients in relation to severity, evolution of the clinical manifestations and immune/inflammatory profile. Methods: This is prospective study recruiting consecutive hospitalized patients with respiratory failure associated with SARS-COV-2 infection and matched controls. After enrollment, patients were assessed every 7 ± 2 days for additional 2 consecutive visits, for a total of 21 days. The severity of the clinical condition was ranked based on the level of respiratory support required. At each time-point blood samples were obtained to assess immune cells and mediators by multiplex immunoassay. Results: Fifty-four COVD-19 and 11 control patients matched for severity were enrolled. At recruitment, lower levels of blood IFN-a were found in COVID-19 patients compared to controls (3.8-fold difference, p < 0.01). Improvements in COVID-19 severity were paralleled by a significant increase of blood IFN-a levels. A significant increase in blood IFN-a was found over the study period in survivors (70% of the study population). A similar trend was found for blood IFN-ß with IFN-ß levels below the threshold of detectability in a substantial proportion of subjects. Significantly higher values of blood lymphocytes and lower levels of IL-10 were found at each time point in patients who survived compared to patients who died. In patients who clinically improved and survived during the study, we found an inverse association between IL-10 and IFN-a levels. Conclusion: The study identifies a blood immune profile defined by deficient IFN-a levels associated with increased IL-10 expression in patients progressing to severe/life threatening COVID-19 conditions, suggesting the involvement of immunological pathways that could be target of pharmacological intervention. Clinical Trial Registration: ClinicalTrials.gov identifier NCT04343053.
|
Open Research Newcastle | |||||||||
| 2021 |
Deliyannis G, Wong CY, McQuilten HA, Bachem A, Clarke M, Jia X, Horrocks K, Zeng W, Girkin J, Scott NE, Londrigan SL, Reading PC, Bartlett NW, Kedzierska K, Brown LE, Mercuri F, Demaison C, Jackson DC, Chua BY, 'TLR2-mediated activation of innate responses in the upper airways confers antiviral protection of the lungs', JCI INSIGHT, 6 (2021) [C1]
The impact of respiratory virus infections on global health is felt not just during a pandemic, but endemic seasonal infections pose an equal and ongoing risk of severe... [more] The impact of respiratory virus infections on global health is felt not just during a pandemic, but endemic seasonal infections pose an equal and ongoing risk of severe disease. Moreover, vaccines and antiviral drugs are not always effective or available for many respiratory viruses. We investigated how induction of effective and appropriate antigen-independent innate immunity in the upper airways can prevent the spread of respiratory virus infection to the vulnerable lower airways. Activation of TLR2, when restricted to the nasal turbinates, resulted in prompt induction of innate immune¿driven antiviral responses through action of cytokines, chemokines, and cellular activity in the upper but not the lower airways. We have defined how nasal epithelial cells and recruitment of macrophages work in concert and play pivotal roles to limit progression of influenza virus to the lungs and sustain protection for up to 7 days. These results reveal underlying mechanisms of how control of viral infection in the upper airways can occur and support the implementation of strategies that can activate TLR2 in nasal passages to provide rapid protection, especially for at-risk populations, against severe respiratory infection when vaccines and antiviral drugs are not always effective or available.
|
Open Research Newcastle | |||||||||
| 2021 |
Girkin J, Loo S-L, Esneau C, Maltby S, Mercuri F, Chua B, Reid AT, Veerati PC, Grainge CL, Wark PAB, Knight D, Jackson D, Demaison C, Bartlett NW, 'TLR2-mediated innate immune priming boosts lung anti-viral immunity', EUROPEAN RESPIRATORY JOURNAL, 58 (2021) [C1]
|
Open Research Newcastle | |||||||||
| 2021 |
Williams TC, Jackson DJ, Maltby S, Walton RP, Ching Y-M, Glanville N, Singanayagam A, Brewins JJ, Clarke D, Hirsman AG, Loo S-L, Wei L, Beale JE, Casolari P, Caramori G, Papi A, Belvisi M, Wark PAB, Johnston SL, Edwards MR, Bartlett NW, 'Rhinovirus-induced CCL17 and CCL22 in Asthma Exacerbations and Differential Regulation by STAT6', AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY, 64, 344-356 (2021) [C1]
|
Open Research Newcastle | |||||||||
| 2021 |
Schuliga M, Kanwal A, Read J, Blokland KEC, Burgess JK, Prele CM, Mutsaers SE, Grainge C, Thomson C, James A, Bartlett NW, Knight DA, 'A cGAS-dependent response links DNA damage and senescence in alveolar epithelial cells: a potential drug target in IPF', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 321, L859-L871 (2021) [C1]
|
Open Research Newcastle | |||||||||
| 2021 |
Proud PC, Tsitoura D, Watson RJ, Chua BY, Aram MJ, Bewley KR, Cavell BE, Cobb R, Dowall S, Fotheringham SA, Ho CMK, Lucas V, Ngabo D, Rayner E, Ryan KA, Slack GS, Thomas S, Wand NI, Yeates P, Demaison C, Zeng W, Holmes I, Jackson DC, Bartlett NW, Mercuri F, Carroll MW, 'Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model', EBioMedicine, 63 (2021) [C1]
|
Open Research Newcastle | |||||||||
| 2021 |
Wark PAB, Pathinayake PS, Kaiko G, Nichol K, Ali A, Chen L, Sutanto EN, Garratt LW, Sohal SS, Lu W, Eapen MS, Oldmeadow C, Bartlett N, Reid A, Veerati P, Hsu AC-Y, Looi K, Iosifidis T, Stick SM, Hansbro PM, Kicic A, 'ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma', RESPIROLOGY, 26, 442-451 (2021) [C1]
Background and objective: COVID-19 is complicated by acute lung injury, and death in some individuals. It is caused by SARS-CoV-2 that requires the ACE2 receptor and se... [more] Background and objective: COVID-19 is complicated by acute lung injury, and death in some individuals. It is caused by SARS-CoV-2 that requires the ACE2 receptor and serine proteases to enter AEC. We determined what factors are associated with ACE2 expression particularly in patients with asthma and COPD. Methods: We obtained lower AEC from 145 people from two independent cohorts, aged 2¿89 years, Newcastle (n = 115) and Perth (n = 30), Australia. The Newcastle cohort was enriched with people with asthma (n = 37) and COPD (n = 38). Gene expression for ACE2 and other genes potentially associated with SARS-CoV-2 cell entry was assessed by qPCR, and protein expression was confirmed with immunohistochemistry on endobronchial biopsies and cultured AEC. Results: Increased gene expression of ACE2 was associated with older age (P = 0.03) and male sex (P = 0.03), but not with pack-years smoked. When we compared gene expression between adults with asthma, COPD and healthy controls, mean ACE2 expression was lower in asthma patients (P = 0.01). Gene expression of furin, a protease that facilitates viral endocytosis, was also lower in patients with asthma (P = 0.02), while ADAM-17, a disintegrin that cleaves ACE2 from the surface, was increased (P = 0.02). ACE2 protein expression was also reduced in endobronchial biopsies from asthma patients. Conclusion: Increased ACE2 expression occurs in older people and males. Asthma patients have reduced expression. Altered ACE2 expression in the lower airway may be an important factor in virus tropism and may in part explain susceptibility factors and why asthma patients are not over-represented in those with COVID-19 complications.
|
Open Research Newcastle | |||||||||
| 2021 |
Collison AM, Sokulsky LA, Kepreotes E, de Siqueira AP, Morten M, Edwards MR, Walton RP, Bartlett NW, Yang M, Nguyen TH, Johnston SL, Foster PS, Mattes J, 'miR-122 promotes virus-induced lung disease by targeting SOCS1', JCI INSIGHT, 6 (2021) [C1]
Virus-induced respiratory tract infections are a major health burden in childhood, and available treatments are supportive rather than disease modifying. Rhinoviruses (... [more] Virus-induced respiratory tract infections are a major health burden in childhood, and available treatments are supportive rather than disease modifying. Rhinoviruses (RVs), the cause of approximately 80% of common colds, are detected in nearly half of all infants with bronchiolitis and the majority of children with an asthma exacerbation. Bronchiolitis in early life is a strong risk factor for the development of asthma. Here, we found that RV infection induced the expression of miRNA 122 (miR-122) in mouse lungs and in human airway epithelial cells. In vivo inhibition specifically in the lung reduced neutrophilic inflammation and CXCL2 expression, boosted innate IFN responses, and ameliorated airway hyperreactivity in the absence and in the presence of allergic lung inflammation. Inhibition of miR-122 in the lung increased the levels of suppressor of cytokine signaling 1 (SOCS1), which is an in vitro-validated target of miR-122. Importantly, gene silencing of SOCS1 in vivo completely reversed the protective effects of miR-122 inhibition on RV-induced lung disease. Higher miR-122 expression in nasopharyngeal aspirates was associated with a longer time on oxygen therapy and a higher rate of treatment failure in 87 infants hospitalized with moderately severe bronchiolitis. These results suggest that miR-122 promotes RV-induced lung disease via suppression of its target SOCS1 in vivo. Higher miR-122 expression was associated with worse clinical outcomes, highlighting the potential use of anti-miR-122 oligonucleotides, successfully trialed for treatment of hepatitis C, as potential therapeutics for RV-induced bronchiolitis and asthma exacerbations.
|
Open Research Newcastle | |||||||||
| 2021 |
Finney LJ, Glanville N, Farne H, Aniscenko J, Fenwick P, Kemp S, Trujillo-Torralbo M-B, Loo SL, Calderazzo MA, Wedzicha JA, Mallia P, Bartlett NW, Johnston SL, Singanayagam A, 'Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon', JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY, 147, 510-+ (2021) [C1]
Background: The mechanisms underlying altered susceptibility and propensity to severe Coronavirus disease 2019 (COVID-19) disease in at-risk groups such as patients wit... [more] Background: The mechanisms underlying altered susceptibility and propensity to severe Coronavirus disease 2019 (COVID-19) disease in at-risk groups such as patients with chronic obstructive pulmonary disease (COPD) are poorly understood. Inhaled corticosteroids (ICSs) are widely used in COPD, but the extent to which these therapies protect or expose patients to risk of severe COVID-19 is unknown. Objective: The aim of this study was to evaluate the effect of ICSs following pulmonary expression of the SARS-CoV-2 viral entry receptor angiotensin-converting enzyme-2 (ACE2). Methods: We evaluated the effect of ICS administration on pulmonary ACE2 expression in vitro in human airway epithelial cell cultures and in vivo in mouse models of ICS administration. Mice deficient in the type I IFN-a/ß receptor (Ifnar1-/-) and administration of exogenous IFN-ß were used to study the functional role of type-I interferon signaling in ACE2 expression. We compared sputum ACE2 expression in patients with COPD stratified according to use or nonuse of ICS. Results: ICS administration attenuated ACE2 expression in mice, an effect that was reversed by exogenous IFN-ß administration, and Ifnar1-/- mice had reduced ACE2 expression, indicating that type I interferon contributes mechanistically to this effect. ICS administration attenuated expression of ACE2 in airway epithelial cell cultures from patients with COPD and in mice with elastase-induced COPD-like changes. Compared with ICS nonusers, patients with COPD who were taking ICSs also had reduced sputum expression of ACE2. Conclusion: ICS therapies in COPD reduce expression of the SARS-CoV-2 entry receptor ACE2. This effect may thus contribute to altered susceptibility to COVID-19 in patients with COPD.
|
Open Research Newcastle | |||||||||
| 2020 |
Sokulsky LA, Garcia-Netto K, Nguyen TH, Girkin JLN, Collison A, Mattes J, Kaiko G, Liu C, Bartlett NW, Yang M, Foster PS, 'A critical role for the CXCL3/CXCL5/CXCR2 neutrophilic chemotactic axis in the regulation of type 2 responses in a model of rhinoviral-induced asthma exacerbation', Journal of Immunology, 205, 2468-2478 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2020 |
Davis JS, Chu G, Pathinayake P, Jones D, Giffard P, Macera L, Choi P, Bartlett NW, 'Seroprevalence of Torque Teno Virus in hemodialysis and renal transplant patients in Australia: A cross-sectional study', TRANSPLANT INFECTIOUS DISEASE, 22 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2020 |
Veerati PC, Mitchel JA, Reid AT, Knight DA, Bartlett NW, Park JA, Grainge CL, 'Airway mechanical compression: Its role in asthma pathogenesis and progression', European Respiratory Review, 29, 1-13 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2020 |
Reid AT, Nichol KS, Veerati PC, Moheimani F, Kicic A, Stick SM, Bartlett NW, Grainge CL, Wark PAB, Hansbro PM, Knight DA, 'Blocking Notch3 Signaling Abolishes MUC5AC Production in Airway Epithelial Cells from Individuals with Asthma', AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY, 62, 513-523 (2020) [C1]
In asthma, goblet cell numbers are increased within the airway epithelium, perpetuating the production of mucus that is more difficult to clear and results in airway mu... [more] In asthma, goblet cell numbers are increased within the airway epithelium, perpetuating the production of mucus that is more difficult to clear and results in airway mucus plugging. Notch1, Notch2, or Notch3, or a combination of these has been shown to influence the differentiation of airway epithelial cells. How the expression of specific Notch isoforms differs in fully differentiated adult asthmatic epithelium and whether Notch influences mucin production after differentiation is currently unknown. We aimed to quantify different Notch isoforms in the airway epithelium of individuals with severe asthma and to examine the impact of Notch signaling on mucin MUC5AC. Human lung sections and primary bronchial epithelial cells from individuals with and without asthma were used in this study. Primary bronchial epithelial cells were differentiated at the air-liquid interface for 28 days. Notch isoform expression was analyzed by Taqman quantitative PCR. Immunohistochemistry was used to localize and quantify Notch isoforms in human airway sections. Notch signaling was inhibited in vitro using dibenzazepine or Notch3-specific siRNA, followed by analysis of MUC5AC. NOTCH3 was highly expressed in asthmatic airway epithelium compared with nonasthmatic epithelium. Dibenzazepine significantly reduced MUC5AC production in air-liquid interface cultures of primary bronchial epithelial cells concomitantly with suppression of NOTCH3 intracellular domain protein. Specific knockdown using NOTCH3 siRNA recapitulated the dibenzazepine-induced reduction in MUC5AC. We demonstrate that NOTCH3 is a regulator of MUC5AC production. Increased NOTCH3 signaling in the asthmatic airway epithelium may therefore be an underlying driver of excess MUC5AC production.
|
Open Research Newcastle | |||||||||
| 2020 |
Loo S-L, Wark PAB, Esneau C, Nichol KS, Hsu AC-Y, Bartlett NW, 'Human coronaviruses 229E and OC43 replicate and induce distinct antiviral responses in differentiated primary human bronchial epithelial cells', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 319, L926-L931 (2020) [C1]
The recurrent emergence of novel, pathogenic coronaviruses (CoVs) severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1; 2002), Middle East respiratory syndrome (... [more] The recurrent emergence of novel, pathogenic coronaviruses (CoVs) severe acute respiratory syndrome coronavirus 1 (SARS-CoV-1; 2002), Middle East respiratory syndrome (MERS)-CoV (2012), and most recently SARS-CoV-2 (2019) has highlighted the need for physiologically informative airway epithelial cell infection models for studying immunity to CoVs and development of antiviral therapies. To address this, we developed an in vitro infection model for two human coronaviruses; alphacoronavirus 229E-CoV (229E) and betacoronavirus OC43-CoV (OC43) in differentiated primary human bronchial epithelial cells (pBECs). Primary BECs from healthy subjects were grown at air-liquid interface (ALI) and infected with 229E or OC43, and replication kinetics and time-course expression of innate immune mediators were assessed. OC43 and 229E-CoVs replicated in differentiated pBECs but displayed distinct replication kinetics: 229E replicated rapidly with viral load peaking at 24 h postinfection, while OC43 replication was slower peaking at 96 h after infection. This was associated with diverse antiviral response profiles defined by increased expression of type I/III interferons and interferon-stimulated genes (ISGs) by 229E compared with no innate immune activation with OC43 infection. Understanding the host-virus interaction for previously established coronaviruses will give insight into pathogenic mechanisms underpinning SARS-CoV-2-induced respiratory disease and other future coronaviruses that may arise from zoonotic sources.
|
Open Research Newcastle | |||||||||
| 2020 |
Veerati PC, Troy NM, Reid AT, Li NF, Nichol KS, Kaur P, Maltby S, Wark PAB, Knight DA, Bosco A, Grainge CL, Bartlett NW, 'Airway Epithelial Cell Immunity Is Delayed During Rhinovirus Infection in Asthma and COPD', FRONTIERS IN IMMUNOLOGY, 11 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2020 |
Kamal F, Glanville N, Xia W, Bakhsoliani E, Aniscenko J, Bartlett NW, Edwards MR, Johnston SL, Singanayagam A, 'Beclomethasone Has Lesser Suppressive Effects on Inflammation and Antibacterial Immunity Than Fluticasone or Budesonide in Experimental Infection Models', Chest, 158, 947-951 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2020 |
Kan S, Hariyadi DM, Grainge C, Knight DA, Bartlett NW, Liang M, 'Airway epithelial-targeted nanoparticles for asthma therapy', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 318, L500-L509 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2020 |
Hondermarck H, Bartlett NW, Nurcombe V, 'The role of growth factor receptors in viral infections: An opportunity for drug repurposing against emerging viral diseases such as COVID-19?', FASEB BIOADVANCES, 2, 296-303 (2020) [C1]
|
Open Research Newcastle | |||||||||
| 2019 |
Singanayagam A, Glanville N, Cuthbertson L, Bartlett NW, Finney LJ, Turek E, Bakhsoliani E, Calderazzo MA, Trujillo-Torralbo M-B, Footitt J, James PL, Fenwick P, Kemp SV, Clarke TB, Wedzicha JA, Edwards MR, Moffatt M, Cookson WO, Mallia P, Johnston SL, 'Inhaled corticosteroid suppression of cathelicidin drives dysbiosis and bacterial infection in chronic obstructive pulmonary disease.', Science translational medicine, 11, 13-13 (2019) [C1]
|
Open Research Newcastle | |||||||||
| 2019 |
McVey MJ, Maishan M, Blokland KEC, Bartlett N, Kuebler WM, 'Extracellular vesicles in lung health, disease, and therapy', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 316, L977-L989 (2019) [C1]
|
Open Research Newcastle | |||||||||
| 2019 |
Waters DW, Blokland KEC, Pathinayake PS, Wei L, Schuliga M, Jaffar J, Westall GP, Hansbro PM, Prele CM, Mutsaers SE, Bartlett NW, Burgess JK, Grainge CL, Knight DA, 'STAT3 Regulates the Onset of Oxidant-induced Senescence in Lung Fibroblasts', AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY, 61, 61-73 (2019) [C1]
|
Open Research Newcastle | |||||||||
| 2019 |
Singanayagam A, Loo S-L, Calderazzo M, Finney LJ, Torralbo M-BT, Bakhsoliani E, Girkin J, Veerati P, Pathinayake PS, Nichol KS, Reid A, Footitt J, Wark PAB, Grainge CL, Johnston SL, Bartlett NW, Mallia P, 'Antiviral immunity is impaired in COPD patients with frequent exacerbations', AMERICAN JOURNAL OF PHYSIOLOGY-LUNG CELLULAR AND MOLECULAR PHYSIOLOGY, 317, L893-L903 (2019) [C1]
Patients with frequent exacerbations represent a chronic obstructive pulmonary disease (COPD) subgroup requiring better treatment options. The aim of this study was to ... [more] Patients with frequent exacerbations represent a chronic obstructive pulmonary disease (COPD) subgroup requiring better treatment options. The aim of this study was to determine the innate immune mechanisms that underlie susceptibility to frequent exacerbations in COPD. We measured sputum expression of immune mediators and bacterial loads in samples from patients with COPD at stable state and during virusassociated exacerbations. In vitro immune responses to rhinovirus infection in differentiated primary bronchial epithelial cells (BECs) sampled from patients with COPD were additionally evaluated. Patients were stratified as frequent exacerbators (=2 exacerbations in the preceding year) or infrequent exacerbators (<2 exacerbations in the preceding year) with comparisons made between these groups. Frequent exacerbators had reduced sputum cell mRNA expression of the antiviral immune mediators type I and III interferons and reduced interferon-stimulated gene (ISG) expression when clinically stable and during virus-associated exacerbation. A role for epithelial cellintrinsic innate immune dysregulation was identified: induction of interferons and ISGs during in vitro rhinovirus (RV) infection was also impaired in differentiated BECs from frequent exacerbators. Frequent exacerbators additionally had increased sputum bacterial loads at 2 wk following virus-associated exacerbation onset. These data implicate deficient airway innate immunity involving epithelial cells in the increased propensity to exacerbations observed in some patients with COPD. Therapeutic approaches to boost innate antimicrobial immunity in the lung could be a viable strategy for prevention and treatment of frequent exacerbations.
|
Open Research Newcastle | |||||||||
| 2018 |
Reid AT, Veerati PC, Gosens R, Bartlett NW, Wark PA, Grainge CL, Stick SM, Kicic A, Moheimani F, Hansbro PM, Knight DA, 'Persistent induction of goblet cell differentiation in the airways: Therapeutic approaches', PHARMACOLOGY & THERAPEUTICS, 185, 155-169 (2018) [C1]
Dysregulated induction of goblet cell differentiation results in excessive production and retention of mucus and is a common feature of several chronic airways diseases... [more] Dysregulated induction of goblet cell differentiation results in excessive production and retention of mucus and is a common feature of several chronic airways diseases. To date, therapeutic strategies to reduce mucus accumulation have focused primarily on altering the properties of the mucus itself, or have aimed to limit the production of mucus-stimulating cytokines. Here we review the current knowledge of key molecular pathways that are dysregulated during persistent goblet cell differentiation and highlights both pre-existing and novel therapeutic strategies to combat this pathology.
|
Open Research Newcastle | |||||||||
| 2018 |
Singanayagam A, Glanville N, Girkin JL, Ching YM, Marcellini A, Porter JD, Toussaint M, Walton RP, Finney LJ, Aniscenko J, Zhu J, Trujillo-Torralbo M-B, Calderazzo MA, Grainge C, Loo S-L, Veerati PC, Pathinayake PS, Nichol KS, Reid AT, James PL, Solari R, Wark PAB, Knight DA, Moffatt MF, Cookson WO, Edwards MR, Mallia P, Bartlett NW, Johnston SL, 'Corticosteroid suppression of antiviral immunity increases bacterial loads and mucus production in COPD exacerbations', NATURE COMMUNICATIONS, 9 (2018) [C1]
|
Open Research Newcastle | |||||||||
| 2018 |
Chairakaki A-D, Saridaki M-I, Pyrillou K, Mouratis M-A, Koltsida O, Walton RP, Bartlett NW, Stavropoulos A, Boon L, Rovina N, Papadopoulos NG, Johnston SL, Andreakos E, 'Plasmacytoid dendritic cells drive acute exacerbations of asthma', Journal of Allergy and Clinical Immunology, 142, 542-556 (2018) [C1]
|
Open Research Newcastle | |||||||||
| 2018 |
Wark PAB, Ramsahai JM, Pathinayake P, Malik B, Bartlett NW, 'Respiratory Viruses and Asthma', SEMINARS IN RESPIRATORY AND CRITICAL CARE MEDICINE, 39, 45-55 (2018) [C1]
Asthma remains the most prevalent chronic respiratory disorder, affecting people of all ages. The relationship between respiratory virus infection and asthma has long b... [more] Asthma remains the most prevalent chronic respiratory disorder, affecting people of all ages. The relationship between respiratory virus infection and asthma has long been recognized, though remains incompletely understood. In this article, we will address key issues around this relationship. These will include the crucial role virus infection plays in early life, as a potential risk factor for the development of asthma and lung disease. We will assess the impact that virus infection has on those with established asthma as a trigger for acute disease and how this may influence asthma throughout life. Finally, we will explore the complex interaction that occurs between the airway and the immune responses that make those with asthma so susceptible to the effects of virus infection.
|
Open Research Newcastle | |||||||||
| 2017 |
Hansel TT, Tunstall T, Trujillo-Torralbo M-B, Shamji B, del-Rosario A, Dhariwal J, Kirk PDW, Stumpf MPH, Koopmann J, Telcian A, Aniscenko J, Gogsadze L, Bakhsoliani E, Stanciu L, Bartlett N, Edwards M, Walton R, Mallia P, Hunt TM, Hunt TL, Hunt DG, Westwick J, Edwards M, Kon OM, Jackson DJ, Johnston SL, 'A Comprehensive Evaluation of Nasal and Bronchial Cytokines and Chemokines Following Experimental Rhinovirus Infection in Allergic Asthma: Increased Interferons (IFN-gamma and IFN-lambda) and Type 2 Inflammation (IL-5 and IL-13)', EBIOMEDICINE, 19, 128-138 (2017) [C1]
Background Rhinovirus infection is a major cause of asthma exacerbations. Objectives We studied nasal and bronchial mucosal inflammatory responses during experimental r... [more] Background Rhinovirus infection is a major cause of asthma exacerbations. Objectives We studied nasal and bronchial mucosal inflammatory responses during experimental rhinovirus-induced asthma exacerbations. Methods We used nasosorption on days 0, 2¿5 and 7 and bronchosorption at baseline and day 4 to sample mucosal lining fluid to investigate airway mucosal responses to rhinovirus infection in patients with allergic asthma (n¿=¿28) and healthy non-atopic controls (n¿=¿11), by using a synthetic absorptive matrix and measuring levels of 34 cytokines and chemokines using a sensitive multiplex assay. Results Following rhinovirus infection asthmatics developed more upper and lower respiratory symptoms and lower peak expiratory flows compared to controls (all P¿<¿0.05). Asthmatics also developed higher nasal lining fluid levels of an anti-viral pathway (including IFN-¿, IFN-¿/IL-29, CXCL11/ITAC, CXCL10/IP10 and IL-15) and a type 2 inflammatory pathway (IL-4, IL-5, IL-13, CCL17/TARC, CCL11/eotaxin, CCL26/eotaxin-3) (area under curve day 0¿7, all P¿<¿0.05). Nasal IL-5 and IL-13 were higher in asthmatics at day 0 (P¿<¿0.01) and levels increased by days 3 and 4 (P¿<¿0.01). A hierarchical correlation matrix of 24 nasal lining fluid cytokine and chemokine levels over 7¿days demonstrated expression of distinct interferon-related and type 2 pathways in asthmatics. In asthmatics IFN-¿, CXCL10/IP10, CXCL11/ITAC, IL-15 and IL-5 increased in bronchial lining fluid following viral infection (all P¿<¿0.05). Conclusions Precision sampling of mucosal lining fluid identifies robust interferon and type 2 responses in the upper and lower airways of asthmatics during an asthma exacerbation. Nasosorption and bronchosorption have potential to define asthma endotypes in stable disease and at exacerbation.
|
||||||||||
| 2017 |
Dhariwal J, Cameron A, Trujillo-Torralbo M-B, Del Rosario A, Bakhsoliani E, Paulsen M, Jackson DJ, Edwards MR, Rana BMJ, Cousins DJ, Hansel TT, Johnston SL, Walton RP, MRC-GSK Strategic Alliance Consortium , 'Mucosal Type 2 Innate Lymphoid Cells Are a Key Component of the Allergic Response to Aeroallergens.', American journal of respiratory and critical care medicine, 195, 1586-1596 (2017) [C1]
|
||||||||||
| 2016 |
Tay H, Wark PAB, Bartlett NW, 'Advances in the treatment of virus-induced asthma', Expert Review of Respiratory Medicine, 10, 629-641 (2016) [C1]
ABSTRACT: Viral exacerbations continue to represent the major burden in terms of morbidity, mortality and health care costs associated with asthma. Those at greatest ri... [more] ABSTRACT: Viral exacerbations continue to represent the major burden in terms of morbidity, mortality and health care costs associated with asthma. Those at greatest risk for acute asthma are those with more severe airways disease and poor asthma control. It is this group with established asthma in whom acute exacerbations triggered by virus infections remain a serious cause of increased morbidity. A range of novel therapies are emerging to treat asthma and in particular target this group with poor disease control, and in most cases their efficacy is now being judged by their ability to reduce the frequency of acute exacerbations. Critical for the development of new treatment approaches is an improved understanding of virus-host interaction in the context of the asthmatic airway. This requires research into the virology of the disease in physiological models in conjunction with detailed phenotypic characterisation of asthma patients to identify targets amenable to therapeutic intervention.
|
Open Research Newcastle | |||||||||
| 2015 |
Jackson DJ, Trujillo-Torralbo M-B, del-Rosario J, Bartlett NW, Edwards MR, Mallia P, Walton RP, Johnston SL, 'The influence of asthma control on the severity of virus-induced asthma exacerbations', JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY, 136, 497-+ (2015) [C3]
|
||||||||||
| 2015 |
Hatchwell L, Collison A, Girkin J, Parsons K, Li J, Zhang J, Phipps S, Knight D, Bartlett NW, Johnston SL, Foster PS, Wark PAB, Mattes J, 'Toll-like receptor 7 governs interferon and inflammatory responses to rhinovirus and is suppressed by IL-5-induced lung eosinophilia', Thorax, 70, 854-861 (2015) [C1]
© 2015 BMJ Publishing Group Ltd & British Thoracic Society.Background Asthma exacerbations represent a significant disease burden and are commonly caused by rhinovi... [more] © 2015 BMJ Publishing Group Ltd & British Thoracic Society.Background Asthma exacerbations represent a significant disease burden and are commonly caused by rhinovirus (RV), which is sensed by Toll-like receptors (TLR) such as TLR7. Some asthmatics have impaired interferon (IFN) responses to RV, but the underlying mechanisms of this clinically relevant observation are poorly understood. Objectives To investigate the importance of intact TLR7 signalling in vivo during RV exacerbation using mouse models of house dust mite (HDM)-induced allergic airways disease exacerbated by a superimposed RV infection. Methods Wild-type and TLR7-deficient (Tlr7<sup>-/-</sup>) BALB/c mice were intranasally sensitised and challenged with HDM prior to infection with RV1B. In some experiments, mice were administered recombinant IFN or adoptively transferred with plasmacytoid dendritic cells (pDC). Results Allergic Tlr7<sup>-/-</sup> mice displayed impaired IFN release upon RV1B infection, increased virus replication and exaggerated eosinophilic inflammation and airways hyper reactivity. Treatment with exogenous IFN or adoptive transfer of TLR7-competent pDCs blocked these exaggerated inflammatory responses and boosted IFN? release in the absence of host TLR7 signalling. TLR7 expression in the lungs was suppressed by allergic inflammation and by interleukin (IL)-5-induced eosinophilia in the absence of allergy. Subjects with moderate-to-severe asthma and eosinophilic but not neutrophilic airways inflammation, despite inhaled steroids, showed reduced TLR7 and IFN?2/3 expression in endobronchial biopsies. Furthermore, TLR7 expression inversely correlated with percentage of sputum eosinophils. Conclusions This implicates IL-5-induced airways eosinophilia as a negative regulator of TLR7 expression and antiviral responses, which provides a molecular mechanism underpinning the effect of eosinophil-targeting treatments for the prevention of asthma exacerbations.
|
Open Research Newcastle | |||||||||
| 2015 |
Singanayagam A, Glanville N, Walton RP, Aniscenko J, Pearson RM, Pinkerton JW, Horvat JC, Hansbro PM, Bartlett NW, Johnston SL, 'A short-term mouse model that reproduces the immunopathological features of rhinovirus-induced exacerbation of COPD', CLINICAL SCIENCE, 129, 245-258 (2015) [C1]
|
Open Research Newcastle | |||||||||
| 2015 |
Girkin J, Hatchwell L, Foster P, Johnston SL, Bartlett N, Collison A, Mattes J, 'CCL7 and IRF-7 Mediate Hallmark Inflammatory and IFN Responses following Rhinovirus 1B Infection', JOURNAL OF IMMUNOLOGY, 194, 4924-4930 (2015) [C1]
Rhinovirus (RV) infections are common and have the potential to exacerbate asthma. We have determined the lung transcriptome in RV strain 1B-infected naive BALB/c mice ... [more] Rhinovirus (RV) infections are common and have the potential to exacerbate asthma. We have determined the lung transcriptome in RV strain 1B-infected naive BALB/c mice (nonallergic) and identified CCL7 and IFN regulatory factor (IRF)-7 among the most upregulated mRNA transcripts in the lung. To investigate their roles we employed anti-CCL7 Abs and an IRF-7-targeting small interfering RNA in vivo. Neutralizing CCL7 or inhibiting IRF-7 limited neutrophil and macrophage influx and IFN responses in nonallergic mice. Neutralizing CCL7 also reduced activation of NF-¿B p65 and p50 subunits, as well as airway hyperreactivity (AHR) in nonallergic mice. However, neither NF-¿B subunit activation nor AHR was abolished with infection of allergic mice after neutralizing CCL7, despite a reduction in the number of neutrophils, macrophages, and eosinophils. IRF-7 small interfering RNA primarily suppressed IFN-a and IFN-b levels during infection of allergic mice. Our data highlight a pivotal role of CCL7 and IRF-7 in RV-induced inflammation and IFN responses and link NF-¿B signaling to the development of AHR.
|
Open Research Newcastle | |||||||||
| 2014 |
Toussaint M, Singanayagam A, Johnston SL, Bartlett N, 'Role Of Interleukine-33 In Rhinovirus-Induced Allergic Asthma Exacerbation', JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY, 133, AB52-AB52 (2014)
|
||||||||||
| 2014 |
Jayaraman A, Bartlett N, Johnston SL, 'Innate and Adaptive Lymphocyte Responses In a Mouse Model Of Rhinovirus-Induced Asthma Exacerbation', JOURNAL OF ALLERGY AND CLINICAL IMMUNOLOGY, 133, AB135-AB135 (2014)
|
||||||||||
| 2013 |
McLean GR, Walton RP, Shetty S, Peel TJ, Paktiawal N, Kebadze T, Gogsadze L, Niespodziana K, Valenta R, Bartlett NW, Johnston SL, 'Corrigendum to: "Rhinovirus infections and immunisation induce cross-serotype reactive antibodies to VP1" [Antiviral Res. 95(3) (2012) 193-201]', Antiviral Research, 97 (2013)
|
||||||||||
| 2013 |
Traub S, Nikonova A, Carruthers A, Dunmore R, Vousden KA, Gogsadze L, Hao W, Zhu Q, Bernard K, Zhu J, Dymond M, McLean GR, Walton RP, Glanville N, Humbles A, Khaitov M, Wells T, Kolbeck R, Leishman AJ, Sleeman MA, Bartlett NW, Johnston SL, 'An Anti-Human ICAM-1 Antibody Inhibits Rhinovirus-Induced Exacerbations of Lung Inflammation', PLoS Pathogens, 9 (2013) [C1]
Human rhinoviruses (HRV) cause the majority of common colds and acute exacerbations of asthma and chronic obstructive pulmonary disease (COPD). Effective therapies are ... [more] Human rhinoviruses (HRV) cause the majority of common colds and acute exacerbations of asthma and chronic obstructive pulmonary disease (COPD). Effective therapies are urgently needed, but no licensed treatments or vaccines currently exist. Of the 100 identified serotypes, ~90% bind domain 1 of human intercellular adhesion molecule-1 (ICAM-1) as their cellular receptor, making this an attractive target for development of therapies; however, ICAM-1 domain 1 is also required for host defence and regulation of cell trafficking, principally via its major ligand LFA-1. Using a mouse anti-human ICAM-1 antibody (14C11) that specifically binds domain 1 of human ICAM-1, we show that 14C11 administered topically or systemically prevented entry of two major groups of rhinoviruses, HRV16 and HRV14, and reduced cellular inflammation, pro-inflammatory cytokine induction and virus load in vivo. 14C11 also reduced cellular inflammation and Th2 cytokine/chemokine production in a model of major group HRV-induced asthma exacerbation. Interestingly, 14C11 did not prevent cell adhesion via human ICAM-1/LFA-1 interactions in vitro, suggesting the epitope targeted by 14C11 was specific for viral entry. Thus a human ICAM-1 domain-1-specific antibody can prevent major group HRV entry and induction of airway inflammation in vivo. © 2013 Traub et al.
|
||||||||||
| 2013 |
Glanville N, Mclean GR, Guy B, Lecouturier V, Berry C, Girerd Y, Gregoire C, Walton RP, Pearson RM, Kebadze T, Burdin N, Bartlett NW, Almond JW, Johnston SL, 'Cross-Serotype Immunity Induced by Immunization with a Conserved Rhinovirus Capsid Protein', PLoS Pathogens, 9 (2013) [C1]
Human rhinovirus (RV) infections are the principle cause of common colds and precipitate asthma and COPD exacerbations. There is currently no RV vaccine, largely due to... [more] Human rhinovirus (RV) infections are the principle cause of common colds and precipitate asthma and COPD exacerbations. There is currently no RV vaccine, largely due to the existence of ~150 strains. We aimed to define highly conserved areas of the RV proteome and test their usefulness as candidate antigens for a broadly cross-reactive vaccine, using a mouse infection model. Regions of the VP0 (VP4+VP2) capsid protein were identified as having high homology across RVs. Immunization with a recombinant VP0 combined with a Th1 promoting adjuvant induced systemic, antigen specific, cross-serotype, cellular and humoral immune responses. Similar cross-reactive responses were observed in the lungs of immunized mice after infection with heterologous RV strains. Immunization enhanced the generation of heterosubtypic neutralizing antibodies and lung memory T cells, and caused more rapid virus clearance. Conserved domains of the RV capsid therefore induce cross-reactive immune responses and represent candidates for a subunit RV vaccine. © 2013 Glanville et al.
|
||||||||||
| 2013 |
Glanville N, Message SD, Walton RP, Pearson RM, Parker HL, Laza-Stanca V, Mallia P, Kebadze T, Contoli M, Kon OM, Papi A, Stanciu LA, Johnston SL, Bartlett NW, '¿dT cells suppress inflammation and disease during rhinovirus-induced asthma exacerbations', Mucosal Immunology, 6, 1091-1100 (2013) [C1]
Most asthma exacerbations are triggered by virus infections, the majority being caused by human rhinoviruses (RV). In mouse models, ¿dT cells have been previously demon... [more] Most asthma exacerbations are triggered by virus infections, the majority being caused by human rhinoviruses (RV). In mouse models, ¿dT cells have been previously demonstrated to influence allergen-driven airways hyper-reactivity (AHR) and can have antiviral activity, implicating them as prime candidates in the pathogenesis of asthma exacerbations. To explore this, we have used human and mouse models of experimental RV-induced asthma exacerbations to examine ¿dT-cell responses and determine their role in the immune response and associated airways disease. In humans, airway ¿dT-cell numbers were increased in asthmatic vs. healthy control subjects during experimental infection. Airway and blood ¿dT-cell numbers were associated with increased airways obstruction and AHR. Airway ¿dT-cell number was also positively correlated with bronchoalveolar lavage (BAL) virus load and BAL eosinophils and lymphocytes during RV infection. Consistent with our observations of RV-induced asthma exacerbations in humans, infection of mice with allergic airways inflammation increased lung ¿dT-cell number and activation. Inhibiting ¿dT-cell responses using anti-¿dTCR (anti-¿dT-cell receptor) antibody treatment in the mouse asthma exacerbation model increased AHR and airway T helper type 2 cell recruitment and eosinophilia, providing evidence that ¿dT cells are negative regulators of airways inflammation and disease in RV-induced asthma exacerbations.
|
||||||||||
| 2013 |
Brignull LM, Czimmerer Z, Saidi H, Daniel B, Villela I, Bartlett NW, Johnston SL, Meira LB, Nagy L, Nohturfft A, 'Reprogramming of lysosomal gene expression by interleukin-4 and Stat6', BMC Genomics, 14 (2013) [C1]
Background: Lysosomes play important roles in multiple aspects of physiology, but the problem of how the transcription of lysosomal genes is coordinated remains incompl... [more] Background: Lysosomes play important roles in multiple aspects of physiology, but the problem of how the transcription of lysosomal genes is coordinated remains incompletely understood. The goal of this study was to illuminate the physiological contexts in which lysosomal genes are coordinately regulated and to identify transcription factors involved in this control.Results: As transcription factors and their target genes are often co-regulated, we performed meta-analyses of array-based expression data to identify regulators whose mRNA profiles are highly correlated with those of a core set of lysosomal genes. Among the ~50 transcription factors that rank highest by this measure, 65% are involved in differentiation or development, and 22% have been implicated in interferon signaling. The most strongly correlated candidate was Stat6, a factor commonly activated by interleukin-4 (IL-4) or IL-13. Publicly available chromatin immunoprecipitation (ChIP) data from alternatively activated mouse macrophages show that lysosomal genes are overrepresented among Stat6-bound targets. Quantification of RNA from wild-type and Stat6-deficient cells indicates that Stat6 promotes the expression of over 100 lysosomal genes, including hydrolases, subunits of the vacuolar H+ ATPase and trafficking factors. While IL-4 inhibits and activates different sets of lysosomal genes, Stat6 mediates only the activating effects of IL-4, by promoting increased expression and by neutralizing undefined inhibitory signals induced by IL-4.Conclusions: The current data establish Stat6 as a broadly acting regulator of lysosomal gene expression in mouse macrophages. Other regulators whose expression correlates with lysosomal genes suggest that lysosome function is frequently re-programmed during differentiation, development and interferon signaling. © 2013 Brignull et al.; licensee BioMed Central Ltd.
|
||||||||||
| 2013 |
Collison AM, Hatchwell LM, Verrills NM, Wark PA, Pereira De Siqueira AL, Tooze MK, Carpenter HC, Don AS, Morris JC, Zimmermann N, Bartlett NW, Rothenberg ME, Johnston SL, Foster PS, Mattes J, 'The E3 ubiquitin ligase midline 1 promotes allergen and rhinovirus-induced asthma by inhibiting protein phosphatase 2A activity', Nature Medicine, 19, 232-237 (2013) [C1]
|
Open Research Newcastle | |||||||||
| 2006 |
Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett N, Kebadze T, Mallia P, Stanciu LA, Parker HL, Slater L, Lewis-Antes A, Kon OM, Holgate ST, Davies DE, Kotenko SV, Papi A, Johnston SL, 'Role of eficient type III interferon-lambda production in asthma exacerbations', Nature Medicine, 12, 1023-1026 (2006) [C1]
|
||||||||||
| Show 101 more journal articles | |||||||||||
Preprint (3 outputs)
| Year | Citation | Altmetrics | Link | |||||
|---|---|---|---|---|---|---|---|---|
| 2022 |
Girkin JLN, Bryant NE, Loo S-L, Hsu A, Williams T, Maltby S, Wark P, Bartlett NW, 'A Mouse Upper Respiratory Tract Coronavirus Infection Model with OC43 Defines Toll-Like Receptor 2/6 Mediated Innate Immune Protection'
|
|||||||
| 2020 |
Wark PAB, Pathinayake P, Kaiko G, Nichol K, Ali A, Chen L, Sutanto E, Garratt L, Sohal S, Lu W, Eapen M, Oldmeadow C, Bartlett N, Reid A, Veerati P, C-Y.Hsu A, Looi K, Iosifidis T, Stick S, Hansbro P, Kicic A, 'ACE2 Expression is elevated in Airway Epithelial Cells from aged and male donors but reduced in asthma' (2020)
|
|||||||
| 2019 |
Singanayagam A, Loo S-L, Calderazzo M, Finney L, Trujillo Torralbo M-B, Bakhsoliani E, Girkin J, Veerati P, Pathinayake P, Nichol K, Reid A, Foottit J, Johnston S, Bartlett N, Mallia P, 'Anti-microbial immunity is impaired in COPD patients with frequent exacerbations' (2019)
|
|||||||
Grants and Funding
Summary
| Number of grants | 49 |
|---|---|
| Total funding | $22,401,084 |
Click on a grant title below to expand the full details for that specific grant.
20251 grants / $833,693
TAKC-02 Inhibition of Rhinovirus-Induced Inflammation in Primary Human Bronchial Epithelial Cells$833,693
Funding body: TAK-Circulator Corporation
| Funding body | TAK-Circulator Corporation |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2025 |
| Funding Finish | 2026 |
| GNo | G2500157 |
| Type Of Funding | C3400 – International For Profit |
| Category | 3400 |
| UON | Y |
20241 grants / $276,000
PREVENT (Pandemic Respiratory Virus survEillaNce Trial)$276,000
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Prof Josh Davis, Prof Nathan Bartlett, Dr Craig Dalton, Dr James Fielding, Dr Adrian Marcato, Peter Massey, Professor Jodie McVernon, Professor Kanta Subbarao |
| Scheme | Partnership Projects |
| Role | Investigator |
| Funding Start | 2024 |
| Funding Finish | 2024 |
| GNo | G2400245 |
| Type Of Funding | Scheme excluded from IGS |
| Category | EXCL |
| UON | Y |
20235 grants / $257,316
Development of antiviral RNA therapeutics targeting SARS-CoV-2 infection$124,666
Funding body: Department of Health and Aged Care
| Funding body | Department of Health and Aged Care |
|---|---|
| Project Team | Assoc Prof Roger Liang, Prof Nathan Bartlett, Professor Anthony Kelleher, Dr Chantelle Ahlenstiel, Professor Maria Kavallaris, Professor Philip Hansbro, Professor Daniela Traini, Professor Pall Thordarson, Associate Professor Kathy Petoumenos, A/P Stuart Turville, Cees van Rijin |
| Scheme | MRFF - COVID-19 Treatment Access and Public Health Activities |
| Role | Investigator |
| Funding Start | 2023 |
| Funding Finish | 2024 |
| GNo | G2200959 |
| Type Of Funding | C1300 - Aust Competitive - Medical Research Future Fund |
| Category | 1300 |
| UON | Y |
TLR2/6 agonist boosting immunogenicity of tumour-targeting RNAi-based nanomedicines$80,000
Funding body: NSW Ministry of Health
| Funding body | NSW Ministry of Health |
|---|---|
| Project Team | Prof Nathan Bartlett, Assoc Prof Roger Liang, Miss Ellie Doubleday, Dr Anna Galkin |
| Scheme | RNA Future Leaders PhD Program |
| Role | Lead |
| Funding Start | 2023 |
| Funding Finish | 2026 |
| GNo | G2200072 |
| Type Of Funding | C2400 – Aust StateTerritoryLocal – Other |
| Category | 2400 |
| UON | Y |
EXercise for Exacerbations and Rhinovirus Treatment (EXERT): human in vitro and mouse in vivo COPD models$27,700
Funding body: Lung Foundation Australia
| Funding body | Lung Foundation Australia |
|---|---|
| Project Team | Prof Nathan Bartlett, Professor Anne Holland |
| Scheme | Early Career Fellowship for Lung Cancer Research |
| Role | Lead |
| Funding Start | 2023 |
| Funding Finish | 2024 |
| GNo | G2300239 |
| Type Of Funding | C1700 - Aust Competitive - Other |
| Category | 1700 |
| UON | Y |
Intranasal lactoferrin to inhibit coronavirus infection$20,001
Funding body: Noumi Operations Pty Ltd
| Funding body | Noumi Operations Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett, Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2023 |
| Funding Finish | 2023 |
| GNo | G2300957 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Improving arbovirus surveillance in the Hunter region through the development of PCR based assay to survey mosquito species and arboviruses.$4,949
Funding body: University of Newcastle
| Funding body | University of Newcastle |
|---|---|
| Project Team | Dr Camille Esneau, Prof Nathan Bartlett, Dr Toby Mills |
| Scheme | Pilot Funding Scheme |
| Role | Investigator |
| Funding Start | 2023 |
| Funding Finish | 2023 |
| GNo | G2300473 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
20226 grants / $275,225
LNR125 anti-IL-25 blocking rhinovirus induced airway neutrophilic inflammation during allergic airways disease exacerbation$135,600
Funding body: Lanier Biotherapeutics, Inc
| Funding body | Lanier Biotherapeutics, Inc |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2201153 |
| Type Of Funding | C3400 – International For Profit |
| Category | 3400 |
| UON | Y |
Lactorferrin (PUREnFERRIN) - In Vitro Studies & POF Model$50,000
Funding body: Pactum Dairy Group Pty Ltd
| Funding body | Pactum Dairy Group Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2101405 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Lactorferrin (PUREnFERRIN) - In Vitro Studies & POF Model$50,000
Funding body: Department of Industry, Science, Energy and Resources
| Funding body | Department of Industry, Science, Energy and Resources |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2101406 |
| Type Of Funding | C2200 - Aust Commonwealth – Other |
| Category | 2200 |
| UON | Y |
Anitviral Efficacy of Exintech Face Masks for Pathogen Mitigation$15,000
Funding body: Exintech Pty Ltd
| Funding body | Exintech Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2200899 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Testing of SARS-CoV-2 inactivation protocols$13,375
Funding body: Avicena Systems Ltd
| Funding body | Avicena Systems Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2200811 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Testing the antiviral efficacy of Dulux coatings for pathogen mitigation$11,250
Funding body: Dulux Group (Australia) Pty Ltd
| Funding body | Dulux Group (Australia) Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2200951 |
| Type Of Funding | Scheme excluded from IGS |
| Category | EXCL |
| UON | Y |
20218 grants / $522,043
Lactoferrin supplementation, Immune Function & Respiratory Virus Infection$199,986
Funding body: Freedom Foods Group Nutritionals Pty Ltd
| Funding body | Freedom Foods Group Nutritionals Pty Ltd |
|---|---|
| Project Team | Prof Lisa Wood, Prof Nathan Bartlett, Dr Bronwyn Berthon, Dr Evan Williams |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Investigator |
| Funding Start | 2021 |
| Funding Finish | 2022 |
| GNo | G2100982 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Efficacy of UV-C devices designed and built by GERMII on known pathogens$61,200
Funding body: Germii Pathogen Mitigation Pty Ltd
| Funding body | Germii Pathogen Mitigation Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2101118 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Assessment of UV lamp sterilisation effectiveness$50,637
Funding body: Fusion HVAC Australia Pty Ltd
| Funding body | Fusion HVAC Australia Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2100685 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Assessment of UV lamp sterilisation effectiveness$50,000
Funding body: Department of Industry, Innovation and Science
| Funding body | Department of Industry, Innovation and Science |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2100739 |
| Type Of Funding | C2200 - Aust Commonwealth – Other |
| Category | 2200 |
| UON | Y |
Lactoferrin supplementation, Immune Function & Respiratory Virus Infection$50,000
Funding body: Department of Industry, Innovation and Science
| Funding body | Department of Industry, Innovation and Science |
|---|---|
| Project Team | Prof Lisa Wood, Prof Nathan Bartlett, Dr Bronwyn Berthon, Dr Evan Williams |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Investigator |
| Funding Start | 2021 |
| Funding Finish | 2022 |
| GNo | G2101004 |
| Type Of Funding | C2200 - Aust Commonwealth – Other |
| Category | 2200 |
| UON | Y |
Airway-targeting anti-viral treatments for COVID-19$49,035
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Nathan Bartlett, A/P Stuart Turville |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2100149 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
Transcriptomics to define host-coronavirus interaction$37,435
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2021 |
| Funding Finish | 2022 |
| GNo | G2101051 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
Pathopen: a Rapid Saliva Point-of-Care Diagnostic for COVID-19$23,750
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Roger Smith, Prof Nathan Bartlett, Prof Paul Dastoor, Doctor Kaushik Maiti |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2021 |
| Funding Finish | 2021 |
| GNo | G2101061 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
20209 grants / $423,439
INNA compounds for the prevention of respiratory virus-induced disease$136,350
Funding body: Ena Respiratory Pty Ltd
| Funding body | Ena Respiratory Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2000959 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Pre-clinical respiratory virus infection models for screening N-myristoyltransferase inhibitors for anti-viral activity$73,600
Funding body: Myricx Pharma Limited
| Funding body | Myricx Pharma Limited |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2000562 |
| Type Of Funding | C3400 – International For Profit |
| Category | 3400 |
| UON | Y |
In vitro studies of PUREnFERRIN™ Lactoferrin$49,418
Funding body: Pactum Dairy Group Pty Ltd
| Funding body | Pactum Dairy Group Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2001275 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
In vitro studies of PUREnFERRIN™ Lactoferrin$49,418
Funding body: Department of Industry, Innovation and Science
| Funding body | Department of Industry, Innovation and Science |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2001362 |
| Type Of Funding | C2200 - Aust Commonwealth – Other |
| Category | 2200 |
| UON | Y |
Zexa Sure Shield Sanitiser coronavirus inactivity research$41,600
Funding body: Sirron Holdings Group Pty Ltd
| Funding body | Sirron Holdings Group Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2000576 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Antiviral Efficacy testing for Silicone (PDMS) embedded with Nano silver$32,020
Funding body: Silfresh Pty Ltd
| Funding body | Silfresh Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2021 |
| GNo | G2001198 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Test Clear Diamond (self-adhesive, silver ion coated plastic film) against coronavirus at various time intervals to determine how quickly and how effective the product is at destroying this virus$24,533
Funding body: Schaffer and Co Pty Ltd
| Funding body | Schaffer and Co Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2000849 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Metis fabric coronavirus inactivating activity$11,500
Funding body: Metis Technologies Pty Ltd
| Funding body | Metis Technologies Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2000976 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
RNAi nanomedicine for coronavirus infection$5,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Nathan Bartlett, Assoc Prof Roger Liang |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2020 |
| GNo | G2000216 |
| Type Of Funding | C3200 – Aust Not-for Profit |
| Category | 3200 |
| UON | Y |
20195 grants / $1,141,005
In vitro human corneal epithelial cell culture models for screening novel AT-API conjugates for the treatment of dry eye disease$940,045
Funding body: Foray Therapeutics Pty Ltd
| Funding body | Foray Therapeutics Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2019 |
| Funding Finish | 2024 |
| GNo | G1900809 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Confirm the mechanism of action (MOA) and determine the efficacy of the Company’s therapeutic INNA-X$50,699
Funding body: Ena Therapeutics Pty Ltd
| Funding body | Ena Therapeutics Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2019 |
| Funding Finish | 2020 |
| GNo | G1901545 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Define the mechanism of action and determine the efficacy of the Company’s therapeutic drug$50,261
Funding body: Ena Therapeutics Pty Ltd
| Funding body | Ena Therapeutics Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett, Dr Su Ling Loo |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2019 |
| Funding Finish | 2019 |
| GNo | G1900110 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Define the mechanism of action and determine the efficacy of the Company’s therapeutic drug$50,000
Funding body: Department of Industry, Innovation and Science
| Funding body | Department of Industry, Innovation and Science |
|---|---|
| Project Team | Prof Nathan Bartlett, Dr Su Ling Loo |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2019 |
| Funding Finish | 2019 |
| GNo | G1900266 |
| Type Of Funding | C2200 - Aust Commonwealth – Other |
| Category | 2200 |
| UON | Y |
Confirm the mechanism of action (MOA) and determine the efficacy of the Company’s therapeutic INNA-X$50,000
Funding body: Department of Industry, Innovation and Science
| Funding body | Department of Industry, Innovation and Science |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Entrepreneurs' Programme: Innovation Connections |
| Role | Lead |
| Funding Start | 2019 |
| Funding Finish | 2020 |
| GNo | G2000024 |
| Type Of Funding | C2200 - Aust Commonwealth – Other |
| Category | 2200 |
| UON | Y |
20181 grants / $1,104,379
How does bronchoconstriction worsen asthma?$1,104,379
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Conj Assoc Prof Christopher Grainge, Prof Nathan Bartlett, Professor Darryl Knight, Con Prof Peter Wark, Professor Alastair Stewart, Stewart, Alastair |
| Scheme | Project Grant |
| Role | Investigator |
| Funding Start | 2018 |
| Funding Finish | 2021 |
| GNo | G1700343 |
| Type Of Funding | C1100 - Aust Competitive - NHMRC |
| Category | 1100 |
| UON | Y |
20174 grants / $1,422,397
Shared innate immune mechanisms underpin-steroid resistant pathogen-induced asthma exacerbations$844,726
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Professor Paul Foster, Assoc Prof Ming Yang, Prof Nathan Bartlett |
| Scheme | Project Grant |
| Role | Investigator |
| Funding Start | 2017 |
| Funding Finish | 2020 |
| GNo | G1600084 |
| Type Of Funding | C1100 - Aust Competitive - NHMRC |
| Category | 1100 |
| UON | Y |
Pre-clinical studies with REG3500 and dupilumab$421,307
Funding body: Sanofi US Services Inc.
| Funding body | Sanofi US Services Inc. |
|---|---|
| Project Team | Prof Nathan Bartlett, Con Prof Peter Wark |
| Scheme | Research Project |
| Role | Lead |
| Funding Start | 2017 |
| Funding Finish | 2019 |
| GNo | G1701373 |
| Type Of Funding | C3400 – International For Profit |
| Category | 3400 |
| UON | Y |
Novel epithelial targets and targeting strategies to prevent asthma exacerbations$136,364
Funding body: Asthma Australia
| Funding body | Asthma Australia |
|---|---|
| Project Team | Prof Nathan Bartlett, Con Prof Peter Wark, Assoc Prof Roger Liang, Professor Darryl Knight |
| Scheme | National Research Program |
| Role | Lead |
| Funding Start | 2017 |
| Funding Finish | 2018 |
| GNo | G1601217 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Plasma Torque Teno Virus load as a novel tool to monitor intensity of immunosuppression in renal transplant recipients$20,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Josh Davis, Prof Nathan Bartlett, Dr Peter Choi |
| Scheme | Project Grant |
| Role | Investigator |
| Funding Start | 2017 |
| Funding Finish | 2017 |
| GNo | G1701566 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
20163 grants / $1,471,783
Inhibition of rhinovirus-induced disease by TLR2 agonist$726,548
Funding body: Ena Therapeutics Pty Ltd
| Funding body | Ena Therapeutics Pty Ltd |
|---|---|
| Project Team | Prof Nathan Bartlett, Con Prof Peter Wark |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2016 |
| Funding Finish | 2019 |
| GNo | G1600747 |
| Type Of Funding | C3100 – Aust For Profit |
| Category | 3100 |
| UON | Y |
Collaborative Research Agreement: New pathways and targets in severe asthma and COPD$611,171
Funding body: Boehringer Ingelheim Pharma GmbH & Co KG
| Funding body | Boehringer Ingelheim Pharma GmbH & Co KG |
|---|---|
| Project Team | Professor Darryl Knight, Conj Assoc Prof Christopher Grainge, Con Prof Peter Wark, Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2016 |
| Funding Finish | 2019 |
| GNo | G1601257 |
| Type Of Funding | C3400 – International For Profit |
| Category | 3400 |
| UON | Y |
Efficacy of ABM109 in blocking rhinovirus induced asthma exacerbation: preclinical proof of concept$134,064
Funding body: Abeome Corporation
| Funding body | Abeome Corporation |
|---|---|
| Project Team | Prof Nathan Bartlett |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2016 |
| Funding Finish | 2016 |
| GNo | G1600775 |
| Type Of Funding | C3400 – International For Profit |
| Category | 3400 |
| UON | Y |
20152 grants / $50,238
Novel anti-viral agent for rhinovirus infection$25,238
Funding body: 3E Therapeutics
| Funding body | 3E Therapeutics |
|---|---|
| Project Team | Nathan Bartlett |
| Scheme | Commercial |
| Role | Lead |
| Funding Start | 2015 |
| Funding Finish | 2015 |
| GNo | |
| Type Of Funding | External |
| Category | EXTE |
| UON | N |
Specifically targeting the airways to prevent virus-induced asthma attacks$25,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Prof Nathan Bartlett, Professor Darryl Knight |
| Scheme | Project Grant |
| Role | Lead |
| Funding Start | 2015 |
| Funding Finish | 2016 |
| GNo | G1501378 |
| Type Of Funding | Grant - Aust Non Government |
| Category | 3AFG |
| UON | Y |
20141 grants / $23,566
Miltenyi Biotec GentleMACS Octo Dissociator with Heaters$23,566
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Professor Phil Hansbro, Professor Paul Foster, Professor Darryl Knight, Prof Dirk Van Helden, Prof Joerg Mattes, Prof Jodie Simpson, Prof Lisa Wood, Prof LIZ Milward, Dr NATHAN Bartlett, Prof Simon Keely, Dr Steven Maltby, Doctor Andrew Jarnicki, Doctor Malcolm Starkey, Assoc Prof Adam Collison, Doctor Shaan Gellatly |
| Scheme | Equipment Grant |
| Role | Investigator |
| Funding Start | 2014 |
| Funding Finish | 2014 |
| GNo | G1500861 |
| Type Of Funding | Other Public Sector - Commonwealth |
| Category | 2OPC |
| UON | Y |
20122 grants / $4,600,000
Mechanisms of interplay between allergy and viruses in asthma$4,000,000
The major cause of asthma attacks are human rhinovirus (RV) infections, these are also related to development of asthma when they occur in early life. We will investigate gene expression and how expression is regulated in lung cells during RV infection to identify molecules/pathways induced by RV infection that are implicated in promoting allergic responses.
We will determine whether RV induction/modification of these molecules/processes are related to pre-existing allergic responses, virus load and clinical severity of asthma exacerbation.
A major cause of asthma is failure of development of immunological tolerance to allergens. We have shown that tolerance can be reversed by a virus infection, but the mechanisms are unknown. We will purify lung cells from the virus-induced breakdown of tolerance model to identify genes implicated in tolerance breakdown and determine whether these are replicated in lung cells in the human.
Finally we will determine whether blocking or inducing molecules implicated in virus induced worsening of allergic responses/breakdown of tolerance, determines severity of RV induced allergic airway inflammation.
Accomplishment of these aims will identify targets for development of novel therapies for asthma and asthma attacks.
Funding body: Medical Research Council of the United Kingdom
| Funding body | Medical Research Council of the United Kingdom |
|---|---|
| Project Team | Sebastian Johnston, Nathan Bartlett, CIs in MRC Centre for Allergic Mechanisms in Asthma |
| Scheme | Program Grant |
| Role | Investigator |
| Funding Start | 2012 |
| Funding Finish | 2017 |
| GNo | |
| Type Of Funding | International - Competitive |
| Category | 3IFA |
| UON | N |
Defining the importance of IL-25 in rhinovirus induced asthma exacerbations$600,000
Rhinovirus infection induces production of IL-25, augmenting Th2-type immune responses and exacerbating allergic airways inflammation. Thus IL-25 plays a central role in the pathogenesis of RV-induced asthma exacerbations and is a good candidate target for the development of new therapies for asthma aexacerbations.
The aim of this application is to investigate rhinovirus-induced IL-25 and define its role in RV-induced asthma exacerbations using a combination of patient tissue analysis and experimental mouse models. If IL-25 expression is induced and related to disease outcomes in the human model, AND causally related to Th2 mediated disease outcomes in the mouse in vivo models this cytokine will be a very strong candidate for immediate translation of approaches to inhibit expression or function in pivotal proof of concept human intervention studies.
Funding body: Medical Research Council of the United Kingdom
| Funding body | Medical Research Council of the United Kingdom |
|---|---|
| Project Team | Nathan Bartlett, Sebastian Johnston |
| Scheme | Project Grant |
| Role | Lead |
| Funding Start | 2012 |
| Funding Finish | 2014 |
| GNo | |
| Type Of Funding | International - Competitive |
| Category | 3IFA |
| UON | N |
20101 grants / $10,000,000
Post infectious immune reprogramming and its association with persistence and chronicity of respiratory allergic diseases (PreDicta)$10,000,000
To analyze the relationship between these infections with persistence of asthma, Predicta will employ the latest technologies of molecular biology, virology, and cytology. The Consortium is privileged to include groups with an excellent track record in clinical research and cohort-based studies that have significantly contributed to the field of allergy and asthma research internationally.
Funding body: European Commission, European Union
| Funding body | European Commission, European Union |
|---|---|
| Project Team | Nikolaos Papadopoulos, Sebastian Johnston, Nathan Bartlett, PreDicta Consortium |
| Scheme | 7th Research Framework Programme |
| Role | Investigator |
| Funding Start | 2010 |
| Funding Finish | 2016 |
| GNo | |
| Type Of Funding | International - Competitive |
| Category | 3IFA |
| UON | N |
Research Supervision
Number of supervisions
Current Supervision
| Commenced | Level of Study | Research Title | Program | Supervisor Type |
|---|---|---|---|---|
| 2025 | PhD | Surveillance and Characterisation of Arboviruses in the Hunter Region | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2023 | PhD | Aptamer Optimisation And Selection For Non-Invasive Monitoring Of Cancer Using A Breathalyser | PhD (Biological Sciences), College of Engineering, Science and Environment, The University of Newcastle | Co-Supervisor |
| 2023 | PhD | TLR2 Agonist Boosting Immunogenicity of Tumour-Targeting RNAi-Based Nanomedicines | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2023 | PhD | Smartphone Aptasensor For The Detection Of Infectious Diseases | PhD (Physics), College of Engineering, Science and Environment, The University of Newcastle | Co-Supervisor |
| 2022 | PhD | Comparative Assessment of Vaccine Technologies across Human Disease Applications | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2022 | PhD | Airway Epithelial Cell-Targeting Nanoparticles to Harness RNA Interference Therapeutics for Respiratory Virus Induced Diseases | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
Past Supervision
| Year | Level of Study | Research Title | Program | Supervisor Type |
|---|---|---|---|---|
| 2025 | PhD | Topical, Sustained Release Drug Delivery Platform for the Treatment of Ocular Pain | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2025 | PhD | Targeting Reactive Oxygen Species in Virus-Induced Airways Disease | PhD (Pharmacy), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2023 | PhD | Soluble Fibre as an Anti-Inflammatory Treatment for Asthma | PhD (Nutritional Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2023 | PhD | The Fibrogenic Actions of IL-25 in Idiopathic Pulmonary Fibrosis (IPF) | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2022 | PhD | Type 2 Innate Lymphoid Cell Dysfunction in Severe Asthma and Response to Treatment with Mepolizumab and Omalizumab | PhD (Medicine), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2022 | PhD | The Role of STATs in the Interaction of Virus and Type 2 Cytokines in Airway Epithelial Cells | PhD (Medical Biochemistry), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2021 | PhD | The Impact of Respiratory Virus Infection on Airway Epithelial Cell Differentiation and Barrier Formation in Asthma | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2021 | PhD | Development of Airway Epithelial Targeted Nanoparticles Loaded with TLR7 agonist for Asthma Therapy | PhD (Pharmacy), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2021 | PhD | The Interplay between Epithelial-derived Type-2 Inflammation and Rhinovirus Infection in Asthma | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2020 | PhD | Rhinovirus Diversity and Replication in Differentiated Airway Epithelial Cells | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Principal Supervisor |
| 2019 | PhD | Role of Mechanical Forces in Asthma Pathogenesis | PhD (Medicine), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2018 | PhD | Innate Anti-Viral Responses of Airway Epithelial Cells to Infection with Rhinovirus and Coronavirus | PhD (Medicine), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2015 | PhD |
Innate Th2 and anti-viral immune responses in rhinovirus-induced asthma exacerbations <p style="margin-bottom:0cm;margin-bottom:.0001pt;text-autospace:none;"><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">W</span><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;"><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">e recently used type I IFN receptor knockout (IFNAR1KO) mice to show that IFN signalling regulates both allergic and innate anti-viral immune responses in a model of RV-induced exacerbation of allergic airways inflammation. In the absence of type I IFN signalling, RV infection profoundly exacerbated allergen-induced Th2 cell activation (Figure 1). This suggests that type I IFN signalling regulates Th2 immune responses during RV infection. However, RV infection did not exacerbate allergic airways inflammation in strain-matched wild type C57 Bl/6 mice, which is in contrast to the model established in the Th2-biased Balb/c strain whereby RV increases allergen-driven inflammation (Bartlett </span><em>et al</em><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">., 2008). Together, this suggests that RV-induced exacerbation of allergic airways inflammation may be dependent on impaired type I IFN signalling and augmented Th2 immunity.&nbsp;</span></span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-autospace:none;"><strong><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">Hypothesis:</span></strong></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-autospace:none;"><strong><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">Type I IFN signalling modulates Th2-mediated allergic airways inflammation during RV-induced asthma exacerbations. </span></strong></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-autospace:none;"><strong><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">Aims:</span></strong><strong><span lang="EN-US" style="font-family:'Times New Roman',serif;"></span></strong></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;"><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">Mouse models of OVA-induced allergic airways inflammation and RV-induced exacerbation of allergic airways inflammation will be used to determine the role of type I IFN signalling in RV-induced asthma exacerbations. </span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-indent:-18.0pt;"><span lang="EN-US" style="font-family:'Times New Roman',serif;"><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">-</span><span style="font-variant-numeric:normal;font-size:7pt;line-height:normal;font-family:'Times New Roman';">&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp; </span></span><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">To characterise and compare innate anti-viral and early Th2 responses in a model of RV-induced asthma exacerbation in both wild type Balb/C and C57 Bl/6 mouse strains.</span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-indent:-18.0pt;"><span lang="EN-US" style="font-family:'Times New Roman',serif;"><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">-</span><span style="font-variant-numeric:normal;font-size:7pt;line-height:normal;font-family:'Times New Roman';">&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp; </span></span><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">To use type I IFN receptor knock out (IFNAR1KO) mice in a model of RV-induced asthma exacerbation to determine how type I IFN regulates Th2 immune responses during allergen challenge and RV infection. </span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-indent:-18.0pt;"><span lang="EN-US" style="font-family:'Times New Roman',serif;"><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">-</span><span style="font-variant-numeric:normal;font-size:7pt;line-height:normal;font-family:'Times New Roman';">&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp; </span></span><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">To investigate the effects of recombinant mouse IFN&beta; treatment in mouse models of asthma and RV-induced asthma exacerbation to determine whether type I IFN signalling can inhibit Th2 immune responses in both the absence and presence of RV infection. </span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;"><span lang="EN-US" style="font-family:'Times New Roman',serif;"><span style="font-family:Arial, Helvetica, sans-serif;font-size:small;"></span></span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;"><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;"><span style="font-family:Arial, Helvetica, sans-serif;font-size:small;">Human </span><em style="font-family:Arial, Helvetica, sans-serif;font-size:small;">in vitro</em><span style="font-family:Arial, Helvetica, sans-serif;font-size:small;"> cell-based assays will be used to investigate the molecular mechanisms of type I IFN signalling.&nbsp;</span></span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-indent:-18.0pt;"><span lang="EN-US" style="font-family:'Times New Roman',serif;"><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">-</span><span style="font-variant-numeric:normal;font-size:7pt;line-height:normal;font-family:'Times New Roman';">&nbsp;&nbsp;&nbsp;</span></span><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">TTo determine the role of type I IFN signalling on epithelial-mediated Th2 immune responses in a model of RV- and IL-4-stimulated bronchial epithelial cell culture. </span></p><p style="margin-bottom:0cm;margin-bottom:.0001pt;text-indent:-18.0pt;"><span lang="EN-US" style="font-family:'Times New Roman',serif;"><span style="font-size:small;font-family:Arial, Helvetica, sans-serif;">-</span><span style="font-variant-numeric:normal;font-size:7pt;line-height:normal;font-family:'Times New Roman';">&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp;&nbsp; </span></span><span lang="EN-US" style="font-family:Arial, Helvetica, sans-serif;font-size:small;">To investigate the mechanisms of type I IFN-mediated regulation Th2 cells in a Th2 polarised CD4+ T cell model.</span></p> |
Medical Science, Imperial College London | Principal Supervisor |
| 2014 | PhD |
Effect of inhaled corticosteroids on viral and bacterial infection in chronic obstructive pulmonary disease Rhinovirus (RV) infections trigger exacerbations of chronic obstructive pulmonary disease (COPD) exacerbations and may precipitate secondary bacterial infections. Inhaled corticosteroids (ICS) are used commonly in COPD but are relatively ineffective in the context of virus-induced exacerbations and may also increase the risk of pneumonia. We hypothesised that, in a mouse model, ICS would suppress anti-viral and anti-bacterial immune responses leading to alteration of the airway microbiota and secondary bacterial infection following RV-induced exacerbation of COPD.<br />Despite extensive optimisation, we were unable to define a representative mouse model of the deficient anti-viral and anti-bacterial responses that are indicative of human COPD. For this reason, and because of difficulties in measuring the airway microbiota in mice, we employed models of primary RV1B and Streptococcus pneumoniae infection as surrogates for viral exacerbation and bacterial colonisation in COPD. Fluticasone propionate (FP) administration prior to RV1B infection suppressed innate and adaptive immune responses leading to impaired virus control, in a dose dependent manner. This effect was causally related to suppression of type I interferon (IFN) as administration of recombinant IFN-β reconstituted IFN-stimulated gene expression and restored virus control. FP suppressed RV-induced airway inflammation but led to enhanced airway mucin production, effects that were unaltered by recombinant IFN-β. FP administration also suppressed innate responses to S. pneumoniae including expression of anti-bacterial cytokines and cathelicidin-related anti-microbial peptide. High dose FP increased lung tissue bacterial loads with the opposite effect observed with lower dose FP despite similar anti-inflammatory effects.<br />Our findings demonstrate beneficial anti-inflammatory effects of ICS during virus-induced COPD exacerbations but reveal some previously unrecognised detrimental effects including increased virus replication and enhanced mucin production. Additionally, we show that high dose ICS administration may increase bacterial loads and thus increase pneumonia risk but lower doses may conversely reduce bacterial loads and therefore could be safer in COPD. |
Medical Science, Imperial College London | Co-Supervisor |
| 2012 | PhD |
The role of IL-25 in rhinovirus-induced asthma exacerbations Background and hypothesis: Rhinovirus (RV) infections are the principal cause of asthma exacerbations. While Th2-mediated inflammation is clearly implicated in the asthmatic response, it is unknown how the immune response to RV infection interacts with Th2 immunity to enhance disease pathogenesis. The epithelial-derived cytokine, IL-25, has been identified as an initiator and regulator of Th2 immunity and plays a role in asthma pathogenesis. Based on the fact that bronchial epithelial cells are the primary site of RV infection, we hypothesized that RV induces IL-25 production providing a link between infection and Th2 driven allergic inflammation. Aims and methods: RV-induced IL-25 expression was measured in human bronchial epithelial cells (HBECs) obtained from bronchoscopic brushings from atopic asthmatics and healthy patients. Mouse models of RV infection and RV-induced allergic airways disease were also employed to examine IL-25 induction in response to RV infection and/or OVA sensitisation and challenge. Finally, to define a mechanistic role for RV-induced IL-25, signalling mediated by IL-25 was blocked in our model of RV-induced allergic airways disease by neutralising the IL-25 receptor. Results: RV-infected HBECs from asthmatics expressed significantly greater IL-25 gene and protein compared with cells from healthy controls. Furthermore, RV infection of mice induced IL-25 expression in the airway epithelium as well as in inflammatory cells in the airway lamina propia. Using a mouse model of RV-induced allergic disease, we demonstrated that RV enhanced allergen-driven IL-25 gene and protein expression which was associated with increased Th2 inflammation in the lung. Finally, by blocking IL-25 signalling in an RV-infected and OVA-sensitised and challenged mouse, several key features of the exacerbation phenotype were significantly reduced including airway leukocyte infiltration, BAL Th2 cytokines and chemokines and Th2 cells. Conclusions: These novel findings indicate that RV-induced IL-25 plays an important role in enhancing Th2 inflammation associated with the exacerbation phenotype which is mediated by binding to the IL-25 receptor. |
Medical Science, Imperial College London | Principal Supervisor |
| 2011 | PhD |
The role of IL-15 in response to rhinovirus infections Rhinoviruses (RV) cause the common cold and are major precipitants of asthma exacerbations. The underlying mechanisms of RV-induced airways disease are unclear. IL-15 is a proinflammatory cytokine produced during viral infections and plays a key role in the regulation of NK cells. Using mouse models of RV infection and RV-induced asthma exacerbation we examined the role of IL-15 and its importance for NK cell responses during RV infections in allergic and non-allergic airways. We demonstrate RV-induced IL-15 upregulation in the airway and lungs of BALB/c mice at day 1 after infection and accumulation of NK cells in the airway and lungs at days 1-2 and 2-4 respectively. The NK cells exhibited an activated phenotype characterised by upregulated CD69, IFN-γ and GranzymeB expression. Blocking IL-15 upon intranasal administration of an IL-15 neutralising antibody inhibited the NK cell response to RV infection, which was associated with deficient IFN-γ production and increased expression of Th2 mediators. IL-15Rα knockout mice lack NK cells and also demonstrated deficient IFN-γ and increased Th2 responses to RV infection; these mice also exhibited deficient CD8+ T cell responses and an increased viral load. Similar results were observed in RV infected IFNAR1 ko mice, which was associated with deficient IL-15 upregulation. We suggest that RV-induced IL-15 is mediated by type I interferon signalling, and is necessary for NK cell responses and early IFN-γ production during RV-1B infection, which drives development of appropriate Th1 antiviral responses. In the absence of this pathway, Th2 responses result and are associated with impaired antiviral immunity. To examine the interaction between allergen driven Th2 immunity and RV infection, we employed a RV-induced asthma exacerbation model. Unexpectedly, RV infected allergen challenged mice, despite having increased viral load, demonstrated increased IL-15 expression and NK cell responses, revealing a novel interaction between allergic responses and antiviral immunity. |
Microbiology, Imperial College London | Co-Supervisor |
| 2010 | PhD |
T cell responses in models of rhinovirus-induced airways disease <p style="text-align:justify;line-height:150%;"><span lang="EN-GB" style="font-family:'Arial',sans-serif;">Human Rhinoviruses (HRV) cause the common cold and are associated wtih more severe respiratory diseases such as asthma exacerbations. There is good evidence that T lymphocytes (T cells) play a central role in the pathogenesis of atopic asthma however little is known about T cell responses to HRV infection or how HRV infection modulates T cell responses in asthma. One T cell subset expresses the γδ TCR and are capable of modulating inflammation & AHR in mouse models of infection and asthma. Furthermore we have observed increased γδ T cells in the airways of human atopic asthmatics following experimental HRV infection which was associated with worse symptoms, enhanced airway inflammation and diminished lung function. Despite the association of γδ T cells with disease in asthma the functional role of these cells is unknown (Message et al., unpublished data). The aims of this thesis were to use recently developed mouse models and characterise the T cell responses to HRV infection in healthy and in ovalbumin (OVA) sensitised & challenged (asthmatic) mice, and to define a role for γδ T cells in RV infection and RV-induced asthma exacerbations... </span></p><p style="line-height:150%;"><span lang="EN-GB">Consistent with observations in human studies, mouse HRV infection was characterised by neutrophilic and lymphocytic airways inflammation. The airway and lung-tissue lymphocyte response to HRV infection was composed of activated CD4+, CD8+ and CD4<sup>-</sup>/CD8<sup>-</sup> γδ TCR+ T cells<span style="color:#999999;"> </span>and was weakly Th1 orientated.<span style="color:#999999;"> </span>In HRV induced asthma exacerbation, HRV infection modestly enhanced numbers of multiple T cell populations in the airway and lung tissue as well as levels of Th2 cytokines such as IL-4. Systemic depletion of γδ T cells with a monoclonal anti-TCRγδ antibody in the HRV infection model modestly enhanced some aspects of airways inflammation such as neutrophil recruitment but had no effect on AHR. In both the asthma and the HRV induced asthma exacerbation models, γδ T cell depletion enhanced AHR at 48hrs post allergen challenge/ HRV infection. In the allergic asthma model γδ T cell depletion also enhanced some aspects of airways inflammation such as… </span></p><p style="text-align:justify;line-height:150%;"><span lang="EN-GB" style="font-family:'Arial',sans-serif;">This thesis has demonstrated that HRV infection is capable of increasing multiple T cell populations and associated cytokine production in the lung, Antibody-mediated depletion studies specifically defined a function in disease for γδ T cells indicating that they perform a regulatory function, limiting both virus and allergen induced airway inflammation and, in asthma and asthma exacerbation models, also limiting AHR.</span></p> |
Medical Studies, Imperial College London | Co-Supervisor |
News
News • 23 Jan 2024
Citizen of the Year honoured for pioneering COVID-19 treatment
Two leading University of Newcastle health researchers, Professor Nathan Bartlett and Emeritus Professor Julie Byles have each been honoured in the 2024 City of Newcastle Citizen of the Year Awards.
News • 7 Oct 2021
COVID-19 nasal spray moves to Phase 2 study
Encouraging preliminary results from an ongoing phase 1 study have triggered the announcement of phase 2 studies for a first-in-class nasal spray to protect people from respiratory viral diseases such as COVID-19 and influenza.
News • 2 Feb 2021
Research shows nasal spray that protects against COVID-19 is also effective against the common cold
Research into a new drug which primes the immune system in the respiratory tract and is in development for COVID-19 shows it is also effective against rhinovirus.
News • 16 Apr 2020
Drug repurposing potential for COVID-19
The COVID-19 pandemic has highlighted the need for anti-viral therapies to treat respiratory virus infections. With a vaccine probably at least 12 months away, drug repurposing (using clinically approved drugs which also have anti-viral activity) offers hope in fast-tracking therapies to possibly treat infected people and save lives.
News • 14 Jun 2018
Research highlights need for a new approach to COPD management
Researchers at the University of Newcastle and Imperial College London have provided evidence that a conventional treatment for common respiratory diseases such as chronic obstructive pulmonary disease (COPD) can actually lead to worse health outcomes when used to treat symptoms caused by respiratory virus infections.
Professor Nathan Bartlett
Position
Associate Dean Industry and Engagement
Bartlett Lab
School of Biomedical Sciences and Pharmacy
College of Health, Medicine and Wellbeing
Contact Details
| nathan.bartlett@newcastle.edu.au | |
| Phone | 0240420171 |
Office
| Room | HMRI2405 |
|---|---|
| Building | Hunter Medical Research Institute |
| Location | John Hunter Hospital Site , |