Dr Steven Maltby
Casual Academic
School of Biomedical Sciences and Pharmacy
- Email:steven.maltby@newcastle.edu.au
- Phone:(02) 404 20173
Getting evidence into the right hands
Once research is completed, Dr Steven Maltby’s work helps to ensure it is communicated effectively to the right people—boosting reach, interest and impact.

After many years in basic research focussed on understanding immune responses, Dr Steven Maltby has recently switched his focus to communicating research findings after discovery.
After all, he reasons, evidence that sits on a dusty shelf has little effect on lives or progress. Drawing on his health research expertise, Steven is now investigating how results can be better communicated to create the greatest impact for specific audiences. For example, improving clinical care for patients or developing new training resources for students.
“Beyond performing research, it’s very important for academics to communicate new knowledge in a language and format that suits the target audience,” says Steven.
“These audiences may be quite diverse, including other researchers and clinicians, students and teaching staff and the general public.”
“My work seeks to make new knowledge and data accessible and more broadly available.”
It starts with research
Steven moved from Canada to Australia in 2012, joining the University of Newcastle’s research community as a postdoctoral research fellow supervised by Laureate Professor Paul Foster. At the time, Steven’s research focused on understanding how bone marrow function changes in response to disease and infection.
“The aim of my studies was to characterise what happens when a virus is first detected by the immune system, including systemic changes in the bone marrow.”
“The bone marrow houses immune cell progenitors that give rise to mature immune cells, as well as structural cells that are important for maintaining mineral bone. The immune response to disease often causes a lot of the damage and pathology.”
Building on understandings gained from Steven’s early research into immune responses, he transitioned into more clinical research to improve treatment of lung disease, especially severe asthma from 2015. By facilitating improved communication of evidence, Steven has given patients and clinicians increased access to the best evidence from which to base treatment decisions.
“My past research focused on understanding how immune responses occur in the body. Building on this, I applied knowledge to the development of technologies for teaching and training, including the Severe Asthma Toolkit, which was developed for clinicians. I managed project development of this resource in my role with The University of Newcastle’s Centre of Excellence in Severe Asthma.”
Disseminating the results
Steven’s approach to best-practice communication has three main steps.
First, identity high-quality knowledge and evidence that needs to be communicated. Second, translate the evidence in a way that will be meaningful for the target audience. Finally, present the information in a way that is useful, accessible, efficient and engaging. Steven acknowledges that this process is not always straight-forward.
“People are constantly bombarded with information and are very time-poor. So, a major challenge is identifying and packaging information in a way that can be readily accessed, and then getting it into the hands of the target audience in a way that they will engage with.”
As a project officer for the University’s Advanced Training Systems group (since 2019) and the Centre of Excellence in Severe Asthma, Steven’s work contributes to the development of several innovative communication resources for academics, professionals and public audiences.
“The communication takes many forms. We’ve created website and social media content and used technologies such as mixed reality (XR), virtual reality (VR), augmented reality (AR) and 360-degree video to communicate the evidence in a way that is practical, tailored and engaging for our audiences.”
“Our work is tailored for a variety of settings. For example, within a university, it supports the development and delivery of efficient and engaging teaching approaches to support understanding of subject matter and the development of practical skills.”
Supporting evidence-based decisions
The Severe Asthma Toolkit is one of Steven’s most recent projects. Working as project manager, in partnership with the Centre of Excellence in Severe Asthma, Steven’s goal was to create a comprehensive asthma resource for clinicians in public and private clinic settings.
“The Severe Asthma Toolkit is a web-based resource that was launched in March 2018 and has now been accessed by more than 20,000 users from over 150 countries. Its aim is to improve people’s understanding of severe asthma and management of the disease.
“Like our other projects, this work is really about presenting data to people in a way that can be understood and is freely accessible, so that research dissemination can happen effectively.”
Steven’s work continues to include a strong research component. His team are always investigating the best designs and approaches for teaching and learning resources. The hope, he says, is to support more individuals to lead more informed lives and make evidence-based decisions.
“I believe it is important that every audience has access the information they need, in a format that suits them, to inform evidence-based decision making. A clear understanding of evidence is required to address any of the major problems that society faces.”
Getting evidence into the right hands
Once research is completed, Dr Steven Maltby’s work helps to ensure it is communicated effectively to the right people—boosting reach, interest and impact.After many years in basic research focussed on understanding immune responses, Dr Steven Maltby has recently switched his…
Career Summary
Biography
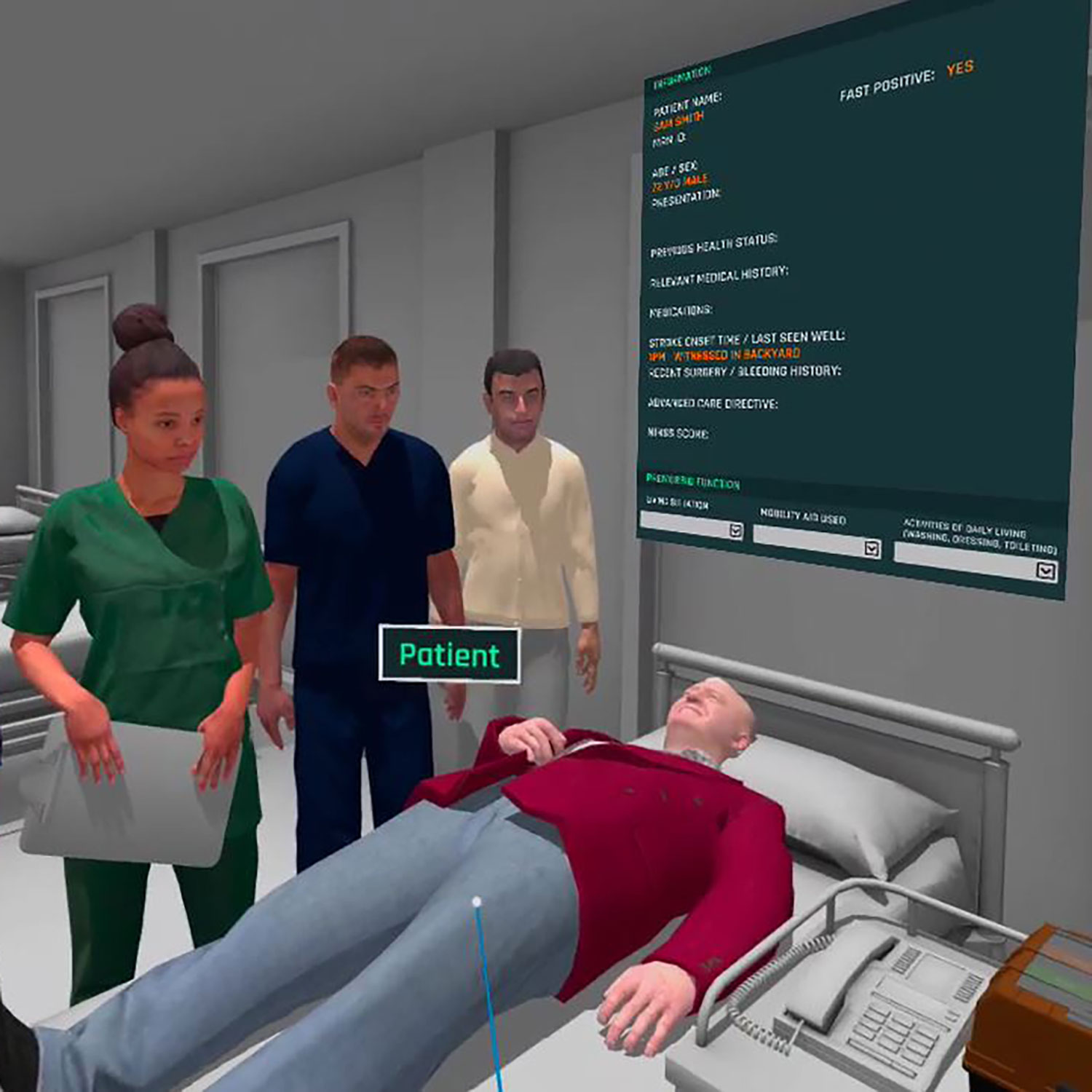
Working with the Centre for Advanced Training Systems led by Prof. Rohan Walker and A/Prof. Eugene Nalivaiko to develop innovative platforms that use virtual and augmented reality (collectively termed mixed reality; XR) for teaching and training. Our projects deliver and assess applications in Defence, Education and Health. As one example of this work, I lead the TACTICS VR project that provides practical training to healthcare professionals on hyper-acute stroke care workflow. Healthcare professionals work through real-world stroke cases and actively make decisions on stroke management with real-time feedback. The 1st TACTICS VR - Stroke Hyper-Acute Management module was assessed in an NHMRC-funded trial at hospitals across 3 Australian states. In partnership with the NSW Agency for Clinical Innovation we expanded the platform with modules for both telehealth and stroke nursing workflow, which are currently deployed at hospitals across NSW. Most recently, we are working with NSW Ambulance to apply this approach to pre-hospital paramedic stroke assessment and transport. The TACTICS VR platform continues to expand, with future modules planned in stroke, cardiac and trauma workflow.
I also work with several UON academics on a casual basis (e.g. A/Prof. Nathan Bartlett), providing writing support for the development of research publications, industry reports, presentations and teaching materials.
Past Experience:
I previously worked as Executive Officer with the Excellence in Severe Asthma (www.severeasthma.org.au; 2015-2019), with Profs. Vanessa McDonald and Peter Gibson. In this role, I was actively involved in science communications, logistics and administration of the research network and development of the Severe Asthma Toolkit, an online evidence-based resource to inform severe asthma management.
I initially came to The University of Newcastle on a Post-doctoral Research Fellowship, with Laureate Prof. Paul Foster. My research focused on characterizing changes in the bone marrow during disease and infection. During a virus infection, an immune response is rapidly induced. This immune response is required to kill the virus and infected cells. However, the immune response often also causes a lot of the damage and pathology that is observed. The aim of my studies was characterise what happens when a virus is first detected by the immune system, including systemic changes in the bone marrow. The bone marrow houses immune cell progentors that give rise to mature immune cells, as well as structural cells that are important for maintaining mineral bone.
I completed PhD studies with Dr Kelly McNagny at The Biomedical Research Centre, University of British Columbia in Vancouver, BC, Canada. My research focused on the role of CD34 (and the related molecule podocalyxin) in immune responses, using mouse models of disease. Those studies identified the importance of CD34 for efficient eosinophil and mast cell migration to sites of inflammation during disease. Further, I demonstrated that loss of CD34 expression (using transgenic Cd34-/- animals) resulted in reduced disease severity in mouse models of asthma and ulcerative colitis.
Research ExpertiseMain Research Focus Areas: Teaching Platforms and Technologies; Medical Education & Skills Training; Virtual Reality; Augmented Reality; Bone Marrow Responses; Immune Cell Activation and Migration; Virus Infections; Asthma & Respiratory Disease
Teaching Expertise
Immunology; Cell Biology; Disease Pathogenesis; Hematopoiesis
Qualifications
- PhD, University of British Columbia - Canada
- Bachelor of Science, University of British Columbia - Canada
Keywords
- Adult Education
- Asthma
- Automated Data Capture
- Bone Marrow
- Disease Models
- Hematopoiesis
- Immunology
- Infection
- Medical Education
- Skills Training
- Virtual Reality
- Virus
- Workplace Training
Languages
- English (Fluent)
Fields of Research
| Code | Description | Percentage |
|---|---|---|
| 460708 | Virtual and mixed reality | 50 |
| 390305 | Professional education and training | 30 |
| 320404 | Cellular immunology | 20 |
Professional Experience
UON Appointment
| Title | Organisation / Department |
|---|---|
| Casual Academic | University of Newcastle School of Biomedical Sciences and Pharmacy Australia |
Academic appointment
| Dates | Title | Organisation / Department |
|---|---|---|
| 1/1/2018 - | Casual Academic | College of Health, Medicine and Wellbeing, University of Newcastle Australia |
| 1/1/2012 - 5/5/2016 |
University of Newcastle Research Fellow University of Newcastle Research Fellowship |
University of Newcastle School of Biomedical Sciences and Pharmacy Australia |
| 1/7/2010 - 1/9/2011 | Post-doctoral fellow | University of British Columbia The Biomedical Research Centre Canada |
Professional appointment
| Dates | Title | Organisation / Department |
|---|---|---|
| 18/7/2020 - | Project Manager | Centre for Advanced Training Systems, The University of Newcastle Australia |
| 18/9/2019 - 18/7/2020 | Project Officer | Centre for Advanced Training Systems, The University of Newcastle Australia |
Awards
Research Award
| Year | Award |
|---|---|
| 2012 |
Postdoctoral Research Fellowship Canadian Institutes of Health Research |
| 2012 |
Postdoctoral Research Fellowship University of Newcastle |
Publications
For publications that are currently unpublished or in-press, details are shown in italics.
Chapter (4 outputs)
| Year | Citation | Altmetrics | Link | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2019 |
Girkin J, Maltby S, Singanayagam A, Bartlett N, Malia P, 'In vivo experimental models of infection and disease', Rhinovirus infections and disease: Rethinking impact on human health and disease, Elsevier, London 195-238 (2019) [B1]
|
Nova | |||||||||
| 2015 |
Maltby S, Plank M, Ptaschinski C, Mattes J, Foster PS, 'MicroRNA function in mast cell biology: Protocols to characterize and modulate MicroRNA expression', Mast Cells: Methods and Protocols, Springer, New York 287-304 (2015) [B1]
|
Nova | |||||||||
| 2013 |
Maltby S, McNagny KM, Ackerman SJ, Du J, Mori Y, Iwasaki H, et al., 'Eosinophilopoiesis', Eosinophils in Health and Disease 73-119 (2013) [B2]
|
||||||||||
| Show 1 more chapter | |||||||||||
Journal article (56 outputs)
| Year | Citation | Altmetrics | Link | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2024 |
Mayall JR, Horvat JC, Mangan NE, Chevalier A, McCarthy H, Hampsey D, et al., 'Interferon-epsilon is a novel regulator of NK cell responses in the uterus', EMBO Molecular Medicine, 16 267-293 [C1]
|
Nova | |||||||||
| 2024 |
Maltby S, Mahadevan JJ, Spratt NJ, Garcia-Esperon C, Kluge MG, Paul CL, et al., 'Implementation and sustainment of virtual reality stroke workflow training for physician trainees at comprehensive stroke centres: a quantitative and qualitative study.', BMC Med Educ, 24 1494 (2024) [C1]
|
||||||||||
| 2023 |
Kluge MG, Maltby S, Kuhne C, Evans DJR, Walker FR, 'Comparing approaches for selection, development, and deployment of extended reality (XR) teaching applications: A case study at The University of Newcastle Australia', EDUCATION AND INFORMATION TECHNOLOGIES, 28 4531-4562 (2023) [C1]
|
Nova | |||||||||
| 2023 |
Girkin JLN, Bryant NE, Loo S-L, Hsu A, Kanwal A, Williams TC, et al., 'Upper Respiratory Tract OC43 Infection Model for Investigating Airway Immune-Modifying Therapies.', Am J Respir Cell Mol Biol, 69 614-622 (2023) [C1]
|
Nova | |||||||||
| 2023 |
Kluge MG, Maltby S, Kuhne C, Walker N, Bennett N, Aidman E, et al., 'Evaluation of a Virtual Reality Platform to Train Stress Management Skills for a Defense Workforce: Multisite, Mixed Methods Feasibility Study.', J Med Internet Res, 25 e46368 (2023) [C1]
|
Nova | |||||||||
| 2023 |
Kuhne C, Kecelioglu ED, Maltby S, Hood RJ, Knott B, Ditton E, et al., 'Direct comparison of virtual reality and 2D delivery on sense of presence, emotional and physiological outcome measures', Frontiers in Virtual Reality, 4 (2023) [C1] Introduction: Virtual-reality (VR) technology has, over the last decade, quickly expanded from gaming into other sectors including training, education, and wellness. One of the mo... [more] Introduction: Virtual-reality (VR) technology has, over the last decade, quickly expanded from gaming into other sectors including training, education, and wellness. One of the most popular justifications for the use of VR over 2D is increased immersion and engagement. However, very little fundamental research has been produced evaluating the comparative impact of immersive VR on the user's cognitive, physiological, and emotional state. Methods: A within-subject cross-over study design was used to directly compare VR and 2D screen delivery of different subject matter content. Both physiological and self-report data were collected for scenes containing calming nature environments, aggressive social confrontations, and neutral content. Results: Compared to 2D, the VR delivery resulted in a higher sense of presence, higher ratings of engagement, fun, and privacy. Confrontational scenes were rated as more tense whilst calming scenes were rated as more relaxing when presented in VR compared to 2D. Physiological data indicated that the scenes promoted overall states of arousal and relaxation in accordance with the scene subject matter (both VR and 2D). However, heart rate (HR) and galvanic skin response (GSR) were consistently higher throughout the VR delivery condition compared to 2D, including responses during scenes of neutral and calming subject matter. Discussion: This discrepancy between emotional and physiological responses for calming and neutral content in VR suggest an elevated arousal response driven by VR immersion that is independent of the emotional and physiological responses to the subject matter itself. These findings have important implications for those looking to develop and utilize VR technology as a training and educational tool as they provide insights into the impact of immersion on the user.
|
Nova | |||||||||
| 2023 |
Maltby S, Garcia-Esperon C, Jackson K, Butcher K, Evans JW, O'Brien W, et al., 'TACTICS VR Stroke Telehealth Virtual Reality Training for Health Care Professionals Involved in Stroke Management at Telestroke Spoke Hospitals: Module Design and Implementation Study.', JMIR Serious Games, 11 e43416 (2023) [C1]
|
Nova | |||||||||
| 2022 |
Kluge MG, Maltby S, Keynes A, Nalivaiko E, Evans DJR, Walker FR, 'Current State and General Perceptions of the Use of Extended Reality (XR) Technology at the University of Newcastle: Interviews and Surveys From Staff and Students', SAGE OPEN, 12 (2022) [C1]
|
Nova | |||||||||
| 2022 |
Girkin JLN, Maltby S, Bartlett NW, 'Toll-like receptor-agonist-based therapies for respiratory viral diseases: thinking outside the cell', EUROPEAN RESPIRATORY REVIEW, 31 (2022) [C1]
|
Nova | |||||||||
| 2021 |
Girkin J, Loo S-L, Esneau C, Maltby S, Mercuri F, Chua B, et al., 'TLR2-mediated innate immune priming boosts lung anti-viral immunity', EUROPEAN RESPIRATORY JOURNAL, 58 (2021) [C1]
|
Nova | |||||||||
| 2021 |
Williams TC, Jackson DJ, Maltby S, Walton RP, Ching Y-M, Glanville N, et al., 'Rhinovirus-induced CCL17 and CCL22 in Asthma Exacerbations and Differential Regulation by STAT6', AMERICAN JOURNAL OF RESPIRATORY CELL AND MOLECULAR BIOLOGY, 64 344-356 (2021) [C1]
|
Nova | |||||||||
| 2021 |
Hood RJ, Maltby S, Keynes A, Kluge MG, Nalivaiko E, Ryan A, et al., 'Development and Pilot Implementation of TACTICS VR: A Virtual Reality-Based Stroke Management Workflow Training Application and Training Framework', FRONTIERS IN NEUROLOGY, 12 (2021) [C1]
|
Nova | |||||||||
| 2021 |
Kluge MG, Maltby S, Walker N, Bennett N, Aidman E, Nalivaiko E, Walker FR, 'Development of a modular stress management platform (Performance Edge VR) and a pilot efficacy trial of a bio-feedback enhanced training module for controlled breathing', PLoS ONE, 16 (2021) [C1] This paper describes the conceptual design of a virtual reality-based stress management training tool and evaluation of the initial prototype in a pilot efficacy study. Performanc... [more] This paper describes the conceptual design of a virtual reality-based stress management training tool and evaluation of the initial prototype in a pilot efficacy study. Performance Edge virtual-reality (VR) was co-developed with the Australian Defence Force (ADF) to address the need for practical stress management training for ADF personnel. The VR application is biofeedback-enabled and contains key stress management techniques derived from acceptance and commitment and cognitive behavioural therapy in a modular framework. End-user-provided feedback on usability, design, and user experience was positive, and particularly complimentary of the respiratory biofeedback functionality. Training of controlled breathing delivered across 3 sessions increased participants' self-reported use of breath control in everyday life and progressively improved controlled breathing skills (objectively assessed as a reduction in breathing rate and variability). Thus the data show that a biofeedback-enabled controlled breathing protocol delivered through Performance Edge VR can produce both behaviour change and objective improvement in breathing metrics. These results confirm the validity of Performance Edge VR platform, and its Controlled Breathing module, as a novel approach to tailoring VR-based applications to train stress management skills in a workplace setting.
|
Nova | |||||||||
| 2021 |
Maltby S, McDonald VM, Upham JW, Bowler SD, Chung LP, Denton EJ, et al., 'Severe asthma assessment, management and the organisation of care in Australia and New Zealand: expert forum roundtable meetings', INTERNAL MEDICINE JOURNAL, 51 169-180 (2021) [C1]
|
Nova | |||||||||
| 2021 |
Barnes JL, Plank MW, Asquith K, Maltby S, Sabino LR, Kaiko GE, et al., 'T-helper 22 cells develop as a distinct lineage from Th17 cells during bacterial infection and phenotypic stability is regulated by T-bet', MUCOSAL IMMUNOLOGY, 14 1077-1087 (2021) [C1]
|
Nova | |||||||||
| 2020 |
Veerati PC, Troy NM, Reid AT, Li NF, Nichol KS, Kaur P, et al., 'Airway Epithelial Cell Immunity Is Delayed During Rhinovirus Infection in Asthma and COPD', FRONTIERS IN IMMUNOLOGY, 11 (2020) [C1]
|
Nova | |||||||||
| 2020 |
Maltby S, Gibson PG, Reddel HK, Smith L, Wark PAB, King GG, et al., 'Severe Asthma Toolkit: an online resource for multidisciplinary health professionals-needs assessment, development process and user analytics with survey feedback', BMJ OPEN, 10 (2020) [C1]
|
Nova | |||||||||
| 2020 |
Hadjigol S, Netto KG, Maltby S, Tay HL, Nguyen TH, Hansbro NG, et al., 'Lipopolysaccharide induces steroid-resistant exacerbations in a mouse model of allergic airway disease collectively through IL-13 and pulmonary macrophage activation', Clinical and Experimental Allergy, 50 82-94 (2020) [C1] Background: Acute exacerbations of asthma represent a major burden of disease and are often caused by respiratory infections. Viral infections are recognized as significant trigge... [more] Background: Acute exacerbations of asthma represent a major burden of disease and are often caused by respiratory infections. Viral infections are recognized as significant triggers of exacerbations; however, less is understood about the how microbial bioproducts such as the endotoxin (lipopolysaccharide (LPS)) trigger episodes. Indeed, increased levels of LPS have been linked to asthma onset, severity and steroid resistance. Objective: The goal of this study was to identify mechanisms underlying bacterial-induced exacerbations by employing LPS as a surrogate for infection. Methods: We developed a mouse model of LPS-induced exacerbation on the background of pre-existing type-2 allergic airway disease (AAD). Results: LPS-induced exacerbation was characterized by steroid-resistant airway hyperresponsiveness (AHR) and an exaggerated inflammatory response distinguished by increased numbers of infiltrating neutrophils/macrophages and elevated production of lung inflammatory cytokines, including TNFa, IFN¿, IL-27 and MCP-1. Expression of the type-2 associated inflammatory factors such as IL-5 and IL-13 were elevated in AAD but not altered by LPS exposure. Furthermore, AHR and airway inflammation were no longer suppressed by corticosteroid (dexamethasone) treatment after LPS exposure. Depletion of pulmonary macrophages by administration of 2-chloroadenosine into the lungs suppressed AHR and reduced IL-13, TNFa and IFN¿ expression. Blocking IL-13 function, through either IL-13-deficiency or administration of specific blocking antibodies, also suppressed AHR and airway inflammation. Conclusions & Clinical Relevance: We present evidence that IL-13 and innate immune pathways (in particular pulmonary macrophages) contribute to LPS-induced exacerbation of pre-existing AAD and provide insight into the complex molecular processes potentially underlying microbial-induced exacerbations.
|
Nova | |||||||||
| 2019 |
Liu G, Mateer SW, Hsu A, Goggins BJ, Tay H, Mathe A, et al., 'Platelet activating factor receptor regulates colitis-induced pulmonary inflammation through the NLRP3 inflammasome', Mucosal Immunology, 12 862-873 (2019) [C1] Extra-intestinal manifestations (EIM) are common in inflammatory bowel disease (IBD). One such EIM is sub-clinical pulmonary inflammation, which occurs in up to 50% of IBD patient... [more] Extra-intestinal manifestations (EIM) are common in inflammatory bowel disease (IBD). One such EIM is sub-clinical pulmonary inflammation, which occurs in up to 50% of IBD patients. In animal models of colitis, pulmonary inflammation is driven by neutrophilic infiltrations, primarily in response to the systemic bacteraemia and increased bacterial load in the lungs. Platelet activating factor receptor (PAFR) plays a critical role in regulating pulmonary responses to infection in conditions, such as chronic obstructive pulmonary disease and asthma. We investigated the role of PAFR in pulmonary EIMs of IBD, using dextran sulfate sodium (DSS) and anti-CD40 murine models of colitis. Both models induced neutrophilic inflammation, with increased TNF and IL-1ß levels, bacterial load and PAFR protein expression in mouse lungs. Antagonism of PAFR decreased lung neutrophilia, TNF, and IL-1ß in an NLRP3 inflammasome-dependent manner. Lipopolysaccharide from phosphorylcholine (ChoP)-positive bacteria induced NLRP3 and caspase-1 proteins in human alveolar epithelial cells, however antagonism of PAFR prevented NLRP3 activation by ChoP. Amoxicillin reduced bacterial populations in the lungs and reduced NLRP3 inflammasome protein levels, but did not reduce PAFR. These data suggest a role for PAFR in microbial pattern recognition and NLRP3 inflammasome signaling in the lung.
|
Nova | |||||||||
| 2018 |
Mateer SW, Mathe A, Bruce J, Liu G, Maltby S, Fricker M, et al., 'IL-6 Drives Neutrophil-Mediated Pulmonary Inflammation Associated with Bacteremia in Murine Models of Colitis', American Journal of Pathology, 188 1625-1639 (2018) [C1] Inflammatory bowel disease (IBD) is associated with several immune-mediated extraintestinal manifestations. More than half of all IBD patients have some form of respiratory pathol... [more] Inflammatory bowel disease (IBD) is associated with several immune-mediated extraintestinal manifestations. More than half of all IBD patients have some form of respiratory pathology, most commonly neutrophil-mediated diseases, such as bronchiectasis and chronic bronchitis. Using murine models of colitis, we aimed to identify the immune mechanisms driving pulmonary manifestations of IBD. We found increased neutrophil numbers in lung tissue associated with the pulmonary vasculature in both trinitrobenzenesulfonic acid¿ and dextran sulfate sodium¿induced models of colitis. Analysis of systemic inflammation identified that neutrophilia was associated with bacteremia and pyrexia in animal models of colitis. We further identified IL-6 as a systemic mediator of neutrophil recruitment from the bone marrow of dextran sulfate sodium animals. Functional inhibition of IL-6 led to reduced systemic and pulmonary neutrophilia, but it did not attenuate established colitis pathology. These data suggest that systemic bacteremia and pyrexia drive IL-6 secretion, which is a critical driver for pulmonary manifestation of IBD. Targeting IL-6 may reduce neutrophil-associated extraintestinal manifestations in IBD patients.
|
Nova | |||||||||
| 2018 |
Maltby S, Lochrin AJ, Bartlett B, Tay HL, Weaver J, Poulton IJ, et al., 'Osteoblasts Are Rapidly Ablated by Virus-Induced Systemic Inflammation following Lymphocytic Choriomeningitis Virus or Pneumonia Virus of Mice Infection in Mice', Journal of Immunology, 200 632-642 (2018) [C1] A link between inflammatory disease and bone loss is now recognized. However, limited data exist on the impact of virus infection on bone loss and regeneration. Bone loss results ... [more] A link between inflammatory disease and bone loss is now recognized. However, limited data exist on the impact of virus infection on bone loss and regeneration. Bone loss results from an imbalance in remodeling, the physiological process whereby the skeleton undergoes continual cycles of formation and resorption. The specific molecular and cellular mechanisms linking virus-induced inflammation to bone loss remain unclear. In the current study, we provide evidence that infection of mice with either lymphocytic choriomeningitis virus (LCMV) or pneumonia virus of mice (PVM) resulted in rapid and substantial loss of osteoblasts from the bone surface. Osteoblast ablation was associated with elevated levels of circulating inflammatory cytokines, including TNF-a, IFN-g, IL-6, and CCL2. Both LCMV and PVM infections resulted in reduced osteoblast-specific gene expression in bone, loss of osteoblasts, and reduced serum markers of bone formation, including osteocalcin and procollagen type 1 N propeptide. Infection of Rag-1-deficient mice (which lack adaptive immune cells) or specific depletion of CD8+ T lymphocytes limited osteoblast loss associated with LCMV infection. By contrast, CD8+ T cell depletion had no apparent impact on osteoblast ablation in association with PVM infection. In summary, our data demonstrate dramatic loss of osteoblasts in response to virus infection and associated systemic inflammation. Further, the inflammatory mechanisms mediating viral infection-induced bone loss depend on the specific inflammatory condition.
|
Nova | |||||||||
| 2018 |
Jones KA, Maltby S, Plank MW, Kluge M, Nilsson M, Foster PS, Walker FR, 'Peripheral immune cells infiltrate into sites of secondary neurodegeneration after ischemic stroke', Brain, Behavior, and Immunity, 67 299-307 (2018) [C1] Experimental stroke leads to microglia activation and progressive neuronal loss at sites of secondary neurodegeneration (SND). These lesions are remote from, but synaptically conn... [more] Experimental stroke leads to microglia activation and progressive neuronal loss at sites of secondary neurodegeneration (SND). These lesions are remote from, but synaptically connected to, primary infarction sites. Previous studies have demonstrated that immune cells are present in sites of infarction in the first hours and days after stroke, and are associated with increased neurodegeneration in peri-infarct regions. However, it is not known whether immune cells are also present in more distal sites where SND occurs. Our study aimed to investigate whether immune cells are present in sites of SND and, if so, how these cell populations compare to those in the peri-infarct zone. Cells were isolated from the thalamus, the main site of SND, and remaining brain tissue 14 days post-stroke. Analysis was performed using flow cytometry to quantify microglia, myeloid cell and lymphocyte numbers. We identified a substantial infiltration of immune cells in the ipsilateral (stroked) compared to the contralateral (control) thalamus, with a significant increase in the percentage of CD4+ and CD8+ T cells. This result was further quantified using immunofluorescent labelling of fixed tissue. In the remaining ipsilateral hemisphere tissue, there were significant increases in the frequency of CD4+ and CD8+ T lymphocytes, B lymphocytes, Ly6G+ neutrophils and both Ly6G-Ly6CLO and Ly6G-Ly6CHI monocytes. Our results indicate that infiltrating immune cells persist in ischemic tissue after the acute ischemic phase, and are increased in sites of SND. Importantly, immune cells have been shown to play pivotal roles in both damage and repair processes after stroke. Our findings indicate that immune cells may also be involved in the pathogenesis of SND and further clinical studies are warranted to characterise the nature of inflammatory cell infiltrates in human disease.
|
Nova | |||||||||
| 2018 |
Porsbjerg C, Sverrild A, Baines KJ, Searles A, Maltby S, Foster PS, et al., 'Advancing the management of obstructive airways diseases through translational research', CLINICAL AND EXPERIMENTAL ALLERGY, 48 493-501 (2018) [C1]
|
Nova | |||||||||
| 2018 |
Nguyen TH, Maltby S, Tay HL, Eyers F, Foster PS, Yang M, 'Identification of IFN- and IL-27 as Critical Regulators of Respiratory Syncytial Virus-Induced Exacerbation of Allergic Airways Disease in a Mouse Model', Journal of Immunology, 200 237-247 (2018) [C1] Respiratory syncytial virus (RSV) infection induces asthma exacerbations, which leads to worsening of clinical symptoms and may result in a sustained decline in lung function. Exa... [more] Respiratory syncytial virus (RSV) infection induces asthma exacerbations, which leads to worsening of clinical symptoms and may result in a sustained decline in lung function. Exacerbations are the main cause of morbidity and mortality associated with asthma, and significantly contribute to asthma-associated healthcare costs. Although glucocorticoids are used to manage exacerbations, some patients respond to them poorly. The underlying mechanisms associated with steroid-resistant exacerbations remain largely unknown. We have previously established a mouse model of RSV-induced exacerbation of allergic airways disease, which mimics hallmark clinical features of asthma. In this study, we have identified key roles for macrophage IFN-¿ and IL-27 in the regulation of RSV-induced exacerbation of allergic airways disease. Production of IFN-¿ and IL-27 was steroid-resistant, and neutralization of IFN-¿ or IL-27 significantly suppressed RSV-induced steroid-resistant airway hyperresponsiveness and airway inflammation. We have previously implicated activation of pulmonary macrophage by TNF-a and/or MCP-1 in the mechanisms of RSV-induced exacerbation. Stimulation of pulmonary macrophages with TNF-a and/or MCP-1 induced expression of both IFN-¿ and IL-27. Our findings highlight critical roles for IFN-¿ and IL-27, downstream of TNF-a and MCP-1, in the mechanism of RSV-induced exacerbation. Thus, targeting the pathways that these factors activate may be a potential therapeutic approach for virus-induced asthma exacerbations.
|
Nova | |||||||||
| 2017 |
Maltby S, Tay HL, Yang M, Foster PS, 'Mouse models of severe asthma: Understanding the mechanisms of steroid resistance, tissue remodelling and disease exacerbation', Respirology, 22 874-885 (2017) [C1] Severe asthma has significant disease burden and results in high healthcare costs. While existing therapies are effective for the majority of asthma patients, treatments for indiv... [more] Severe asthma has significant disease burden and results in high healthcare costs. While existing therapies are effective for the majority of asthma patients, treatments for individuals with severe asthma are often ineffective. Mouse models are useful to identify mechanisms underlying disease pathogenesis and for the preclinical assessment of new therapies. In fact, existing mouse models have contributed significantly to our understanding of allergic/eosinophilic phenotypes of asthma and facilitated the development of novel targeted therapies (e.g. anti-IL-5 and anti-IgE). These therapies are effective in relevant subsets of severe asthma patients. Unfortunately, non-allergic/non-eosinophilic asthma, steroid resistance and disease exacerbation remain areas of unmet clinical need. No mouse model encompasses all features of severe asthma. However, mouse models can provide insight into pathogenic pathways that are relevant to severe asthma. In this review, as examples, we highlight models relevant to understanding steroid resistance, chronic tissue remodelling and disease exacerbation. Although these models highlight the complexity of the immune pathways that may underlie severe asthma, they also provide insight into new potential therapeutic approaches.
|
Nova | |||||||||
| 2017 |
McDonald VM, Maltby S, Reddel HK, King GG, Wark PAB, Smith L, et al., 'Severe asthma: Current management, targeted therapies and future directions A roundtable report', Respirology, 22 53-60 (2017) [C1] Asthma is a chronic respiratory disease characterized by respiratory symptoms, airway inflammation, airway obstruction and airway hyper-responsiveness. Asthma is common and direct... [more] Asthma is a chronic respiratory disease characterized by respiratory symptoms, airway inflammation, airway obstruction and airway hyper-responsiveness. Asthma is common and directly affects 10% of Australians, 1¿5% of adults in Asia and 300 million people worldwide. It is a heterogeneous disorder with many clinical, molecular, biological and pathophysiological phenotypes. Current management strategies successfully treat the majority of patients with asthma who have access to them. However, there is a subset of an estimated 5¿10% of patients with asthma who have severe disease and are disproportionately impacted by symptoms, exacerbations and overall illness burden. The care required for this relatively small proportion of patients is also significant and has a major impact on the healthcare system. A number of new therapies that hold promise for severe asthma are currently in clinical trials or are entering the Australian and international market. However, recognition of severe asthma in clinical practice is variable, and there is little consensus on the best models of care or how to integrate emerging and often costly therapies into current practice. In this article, we report on roundtable discussions held with severe asthma experts from around Australia, and make recommendations about approaches for better patient diagnosis and assessment. We assess current models of care for patient management and discuss how approaches may be optimized to improve patient outcomes. Finally, we propose mechanisms to assess new therapies and how to best integrate these approaches into future treatment.
|
Nova | |||||||||
| 2017 |
Maltby S, Gibson PG, Powell H, McDonald VM, 'Omalizumab Treatment Response in a Population With Severe Allergic Asthma and Overlapping COPD', Chest, 151 78-89 (2017) [C1] Background Asthma and COPD are common airway diseases. Individuals with overlapping asthma and COPD experience increased health impairment and severe disease exacerbations. Effica... [more] Background Asthma and COPD are common airway diseases. Individuals with overlapping asthma and COPD experience increased health impairment and severe disease exacerbations. Efficacious treatment options are required for this population. Omalizumab (anti-IgE) therapy is effective in patients with severe persistent asthma, but limited data are available on efficacy in populations with overlapping asthma and COPD. Methods Data from the Australian Xolair Registry were used to compare treatment responses in individuals with asthma-COPD overlap with responses in patients with severe asthma alone. Participants were assessed at baseline and after 6¿months of omalizumab treatment. We used several different definitions of asthma-COPD overlap. First, we compared participants with a previous physician diagnosis of COPD to participants with no COPD diagnosis. We then made¿comparisons based on baseline lung function, comparing participants with an FEV1 <¿80%¿predicted to those with an FEV1 > 80%¿predicted after bronchodilator use. In the population with an FEV1< 80%, analysis was further stratified based on smoking history. Results Omalizumab treatment markedly improved asthma control and health-related quality of life in all populations assessed based on the Asthma Control Questionnaire and Asthma Quality of Life Questionnaire scores. Omalizumab treatment did not improve lung function (FEV1, FVC, or FEV1/FVC ratio) in populations that were enriched for asthma-COPD overlap (diagnosis of COPD or FEV1¿< 80%/ever smokers). Conclusions Our study suggests that omalizumab improves asthma control and health-related quality of life in individuals with severe allergic asthma and overlapping COPD. These findings provide real-world efficacy data for this patient population and suggest that omalizumab is useful in the management of severe asthma with COPD overlap.
|
Nova | |||||||||
| 2017 |
Plank MW, Kaiko GE, Maltby S, Weaver J, Tay HL, Shen W, et al., 'Th22 Cells Form a Distinct Th Lineage from Th17 Cells In Vitro with Unique Transcriptional Properties and Tbet-Dependent Th1 Plasticity.', JOURNAL OF IMMUNOLOGY, 198 2182-2190 (2017) [C1]
|
Nova | |||||||||
| 2017 |
Foster PS, Maltby S, Rosenberg HF, Tay HL, Hogan SP, Collison AM, et al., 'Modeling T
|
Nova | |||||||||
| 2016 |
Grainge CL, Maltby S, Gibson PG, Wark PAB, McDonald VM, 'Targeted therapeutics for severe refractory asthma: monoclonal antibodies', EXPERT REVIEW OF CLINICAL PHARMACOLOGY, 9 927-941 (2016) [C1]
|
Nova | |||||||||
| 2016 |
Nguyen TH, Maltby S, Simpson JL, Eyers F, Baines KJ, Gibson PG, et al., 'TNF-a and macrophages are critical for respiratory syncytial virus-induced exacerbations in a mouse model of allergic airways disease', Journal of Immunology, 196 3547-3558 (2016) [C1] Viral respiratory infections trigger severe exacerbations of asthma, worsen disease symptoms, and impair lung function. To investigate the mechanisms underlying viral exacerbation... [more] Viral respiratory infections trigger severe exacerbations of asthma, worsen disease symptoms, and impair lung function. To investigate the mechanisms underlying viral exacerbation, we established a mouse model of respiratory syncytial virus (RSV)-induced exacerbation after allergen sensitization and challenge. RSV infection of OVA-sensitized/challenged BALB/c mice resulted in significantly increased airway hyperresponsiveness (AHR) and macrophage and neutrophil lung infiltration. Exacerbation was accompanied by increased levels of inflammatory cytokines (including TNF-a, MCP-1, and keratinocyte-derived protein chemokine [KC]) compared with uninfected OVA-treated mice or OVA-treated mice exposed to UV-inactivated RSV. Dexamethasone treatment completely inhibited all features of allergic disease, including AHR and eosinophil infiltration, in uninfected OVAsensitized/challenged mice. Conversely, dexamethasone treatment following RSV-induced exacerbation only partially suppressed AHR and failed to dampen macrophage and neutrophil infiltration or inflammatory cytokine production (TNF-a, MCP-1, and KC). This mimics clinical observations in patients with exacerbations, which is associated with increased neutrophils and often poorly responds to corticosteroid therapy. Interestingly, we also observed increased TNF-a levels in sputum samples from patients with neutrophilic asthma. Although RSV-induced exacerbation was resistant to steroid treatment, inhibition of TNF-a and MCP-1 function or depletion of macrophages suppressed features of disease, including AHR and macrophage and neutrophil infiltration. Our findings highlight critical roles for macrophages and inflammatory cytokines (including TNF-a and MCP-1) in viral-induced exacerbation of asthma and suggest examination of these pathways as novel therapeutic approaches for disease management.
|
Nova | |||||||||
| 2016 |
Maltby S, Plank M, Tay HL, Collison A, Foster PS, 'Targeting MicroRNA function in respiratory diseases: Mini-review', Frontiers in Physiology, 7 (2016) [C1]
|
Nova | |||||||||
| 2016 |
Wark PAB, Hew M, Maltby S, McDonald VM, Gibson PG, 'Diagnosis and investigation in the severe asthma clinic.', Expert Rev Respir Med, 10 491-503 (2016) [C1]
|
Nova | |||||||||
| 2016 |
Thi HN, Maltby S, Eyers F, Foster PS, Yang M, 'Bromodomain and Extra Terminal (BET) Inhibitor Suppresses Macrophage-Driven Steroid-Resistant Exacerbations of Airway Hyper-Responsiveness and Inflammation', PLOS ONE, 11 (2016) [C1]
|
Nova | |||||||||
| 2015 |
Mateer SW, Maltby S, Marks E, Foster PS, Horvat JC, Hansbro PM, Keely S, 'Potential mechanisms regulating pulmonary pathology in inflammatory bowel disease.', J Leukoc Biol, 98 727-737 (2015) [C1]
|
Nova | |||||||||
| 2015 |
Tay HL, Kaiko GE, Plank M, Li J, Maltby S, Essilfie A-T, et al., 'Correction: Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable Haemophilus Influenzae (NTHi) from Infected Lung.', PLoS pathogens, 11 e1004956 (2015) [O1]
|
||||||||||
| 2015 |
Li JJ, Tay HL, Maltby S, Xiang Y, Eyers F, Hatchwell L, et al., 'MicroRNA-9 regulates steroid-resistant airway hyperresponsiveness by reducing protein phosphatase 2A activity', Journal of Allergy and Clinical Immunology, 136 462-473 (2015) [C1] Background Steroid-resistant asthma is a major clinical problem that is linked to activation of innate immune cells. Levels of IFN-¿ and LPS are often increased in these patients.... [more] Background Steroid-resistant asthma is a major clinical problem that is linked to activation of innate immune cells. Levels of IFN-¿ and LPS are often increased in these patients. Cooperative signaling between IFN-¿/LPS induces macrophage-dependent steroid-resistant airway hyperresponsiveness (AHR) in mouse models. MicroRNAs (miRs) are small noncoding RNAs that regulate the function of innate immune cells by controlling mRNA stability and translation. Their role in regulating glucocorticoid responsiveness and AHR remains unexplored. Objective IFN-¿ and LPS synergistically increase the expression of miR-9 in macrophages and lung tissue, suggesting a role in the mechanisms of steroid resistance. Here we demonstrate the role of miR-9 in IFN-¿/LPS-induced inhibition of dexamethasone (DEX) signaling in macrophages and in induction of steroid-resistant AHR. Methods MiRNA-9 expression was assessed by means of quantitative RT-PCR. Putative miR-9 targets were determined in silico and confirmed in luciferase reporter assays. miR-9 function was inhibited with sequence-specific antagomirs. The efficacy of DEX was assessed by quantifying glucocorticoid receptor (GR) cellular localization, protein phosphatase 2A (PP2A) activity, and AHR. Results Exposure of pulmonary macrophages to IFN-¿/LPS synergistically induced miR-9 expression; reduced levels of its target transcript, protein phosphatase 2 regulatory subunit B (B56) d isoform; attenuated PP2A activity; and inhibited DEX-induced GR nuclear translocation. Inhibition of miR-9 increased both PP2A activity and GR nuclear translocation in macrophages and restored steroid sensitivity in multiple models of steroid-resistant AHR. Pharmacologic activation of PP2A restored DEX efficacy and inhibited AHR. MiR-9 expression was increased in sputum of patients with neutrophilic but not those with eosinophilic asthma. Conclusion MiR-9 regulates GR signaling and steroid-resistant AHR. Targeting miR-9 function might be a novel approach for the treatment of steroid-resistant asthma.
|
Nova | |||||||||
| 2015 |
Plank MW, Maltby S, Tay HL, Stewart J, Eyers F, Hansbro PM, Foster PS, 'MicroRNA Expression Is Altered in an Ovalbumin-Induced Asthma Model and Targeting miR-155 with Antagomirs Reveals Cellular Specificity.', PloS one, 10 1-25 (2015) [C1]
|
Nova | |||||||||
| 2015 |
Tay HL, Kaiko GE, Plank M, Li JJ, Maltby S, Essilfie AT, et al., 'Antagonism of miR-328 Increases the Antimicrobial Function of Macrophages and Neutrophils and Rapid Clearance of Non-typeable Haemophilus Influenzae (NTHi) from Infected Lung', PLoS Pathogens, 11 (2015) [C1] Pathogenic bacterial infections of the lung are life threatening and underpin chronic lung diseases. Current treatments are often ineffective potentially due to increasing antibio... [more] Pathogenic bacterial infections of the lung are life threatening and underpin chronic lung diseases. Current treatments are often ineffective potentially due to increasing antibiotic resistance and impairment of innate immunity by disease processes and steroid therapy. Manipulation miRNA directly regulating anti-microbial machinery of the innate immune system may boost host defence responses. Here we demonstrate that miR-328 is a key element of the host response to pulmonary infection with non-typeable haemophilus influenzae and pharmacological inhibition in mouse and human macrophages augments phagocytosis, the production of reactive oxygen species, and microbicidal activity. Moreover, inhibition of miR-328 in respiratory models of infection, steroid-induced immunosuppression, and smoke-induced emphysema enhances bacterial clearance. Thus, miRNA pathways can be targeted in the lung to enhance host defence against a clinically relevant microbial infection and offer a potential new anti-microbial approach for the treatment of respiratory diseases.
|
Nova | |||||||||
| 2014 |
Maltby S, Hansbro NG, Tay HL, Stewart J, Plank M, Donges B, et al., 'Production and differentiation of myeloid cells driven by proinflammatory cytokines in response to acute pneumovirus infection in mice.', J Immunol, 193 4072-4082 (2014) [C1]
|
Nova | |||||||||
| 2013 |
Plank M, Maltby S, Mattes J, Foster PS, 'Targeting translational control as a novel way to treat inflammatory disease: The emerging role of MicroRNAs', Clinical and Experimental Allergy, 43 981-999 (2013) [C1] Chronic inflammatory diseases (e.g. asthma and chronic obstructive pulmonary disease) are leading causes of morbidity and mortality world-wide and effective treatments are limited... [more] Chronic inflammatory diseases (e.g. asthma and chronic obstructive pulmonary disease) are leading causes of morbidity and mortality world-wide and effective treatments are limited. These disorders can often be attributed to abnormal immune responses to environmental stimuli and infections. Mechanisms leading to inflammation are complex, resulting from interactions of structural cells and activation of both the adaptive and innate arms of the immune system. The activation of structural and immune cells involves both temporary and permanent changes in gene expression in these cells, which underpin chronic inflammation and tissue dysfunction. miRNAs are small non-coding RNAs increasingly being recognized to play important roles in the post-transcriptional regulation of gene expression in mammalian cells by regulating translation. Individual miRNAs can exert their effects by directly inhibiting the translation or stability of multiple mRNAs simultaneously. Thus, the expression or blockade of function of a single miRNA (miR) can result in pronounced alterations in protein expression within a given cell. Dysregulation of miRNA expression may subsequently alter cellular function, and in certain situations predispose to disease. Our current understanding of the role of miRNA in the regulation of inflammatory disease (e.g. allergic diseases) remains limited. In this review, we provide an overview of the current understanding of miRNA biogenesis and function, the roles miRNA play in the regulation of immune cell function and their potential contribution to inflammatory diseases. We also highlight strategies to alter miRNA function for experimental or therapeutic gain, and discuss the potential utility and limitations of targeting these molecules as anti-inflammatory strategies. © 2013 John Wiley & Sons Ltd.
|
Nova | |||||||||
| 2013 |
Plank M, Maltby S, Mattes J, Foster PS, 'Targeting translational control as a novel way to treat inflammatory disease: the emerging role of microRNAs.', Clinical and Experimental Allergy, 43 981-999 (2013)
|
Nova | |||||||||
| Show 53 more journal articles | |||||||||||
Review (1 outputs)
| Year | Citation | Altmetrics | Link | ||
|---|---|---|---|---|---|
| 2009 |
Maltby SJ, Khash K, McNagny KM, 'Mast cells in tumor growth: angiogenesis, tissue remodeling and immune-modulation', Biochimica et biophysica acta - Reviews on Cancer (2009) [D1]
|
Conference (27 outputs)
| Year | Citation | Altmetrics | Link | |||||
|---|---|---|---|---|---|---|---|---|
| 2023 |
Barnes J, Plank M, Asquith K, Maltby S, Rodrigues SL, Kaiko G, et al., 'T-helper-22 cells develop independently of Th17 cells during bacterial infection', RESPIROLOGY (2023)
|
|||||||
| 2023 |
Maltby S, Walker R, 'TACTICS VR: Multi-audience Virtual Reality Workflow Training for Hyper-Acute Stroke Care', INTERNATIONAL JOURNAL OF STROKE (2023)
|
|||||||
| 2022 |
Maltby S, Hood R, Keynes A, Kluge M, Nalivaiko E, Ryan A, et al., 'Ongoing implementation of TACTICS VR: virtual reality-based acute stroke care workflow training', INTERNATIONAL JOURNAL OF STROKE (2022)
|
|||||||
| 2015 |
Plank M, Kaiko G, Maltby S, Tay H, Stewart J, Durum S, Foster P, 'Mapping the cellular source and role of IL-22 in murine lung infections', EUROPEAN RESPIRATORY JOURNAL (2015)
|
|||||||
| 2015 |
Tay H, Kaiko G, Plank M, Li J, Essilfie A, Maltby S, et al., 'THE ROLE OF MIR-328 IN RESPIRATORY DISEASES', RESPIROLOGY, Queensland, AUSTRALIA (2015) [E3]
|
|||||||
| 2015 |
Mateer S, Marks E, Maltby S, Goggins B, Horvat J, Hansbro P, Keely S, 'Pulmonary retention of PMN attracts primed intestinal lymphocytes in a mouse model of inflammatory bowel disease', FASEB JOURNAL (2015) [E3]
|
|||||||
| 2014 |
Mateer S, Maltby S, Marks E, Goggins B, Horvat J, Hansbro P, Keely S, 'Immune cell mis-homing drives secondary organ inflammation in inflammatory bowel disease; a focus on the respiratory system', JOURNAL OF GASTROENTEROLOGY AND HEPATOLOGY (2014) [E3]
|
|||||||
| Show 24 more conferences | ||||||||
Preprint (1 outputs)
| Year | Citation | Altmetrics | Link | |||||
|---|---|---|---|---|---|---|---|---|
| 2023 |
Kluge MG, Maltby S, Kuhne C, Walker N, Bennett N, Aidman E, et al., 'Evaluation of a Virtual Reality Platform to Train Stress Management Skills for a Defense Workforce: Multisite, Mixed Methods Feasibility Study (Preprint) (2023)
|
|||||||
Grants and Funding
Summary
| Number of grants | 21 |
|---|---|
| Total funding | $1,441,768 |
Click on a grant title below to expand the full details for that specific grant.
Highlighted grants and funding
Utilising telemedicine to increase treatment rates and improve clinical outcomes for acute stroke patients$254,000
Funding body: Hunter New England Health
| Funding body | Hunter New England Health |
|---|---|
| Project Team | Prof Neil Spratt, Doctor Carlos Garcia-Esperon, Prof Rohan Walker, Prof Chris Levi, Prof Jennifer Majersik, Ass Prof Andrew Southerland, Dr Chris Oldmeadow, AProf Kenny Lawson, Prof Christine Paul, Rosemary Carney, Dr Steven Maltby |
| Scheme | HMRI Advanced Out of Hospital Care (AOHC) – Telehealth Research Initiative, Part C |
| Role | Investigator |
| Funding Start | 2021 |
| Funding Finish | 2023 |
| GNo | |
| Type Of Funding | Other Public Sector - State |
| Category | 2OPS |
| UON | N |
20242 grants / $109,310
Incorporating Telestroke (live video feed) at paramedic point of care to increase treatment rates and improve clinical outcomes for acute stroke patients (“At It +” study)$80,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Professor Rohan Walker, Professor Neil Spratt, Doctor Steven Maltby, Conjoint Associate Professor Carlos Garcia Esperon |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2024 |
| Funding Finish | 2024 |
| GNo | G2400325 |
| Type Of Funding | C3300 – Aust Philanthropy |
| Category | 3300 |
| UON | Y |
Collaborative education support on advanced imaging and reperfusion therapy for stroke: Evaluation the effectiveness of a virtual reality-based training platform in improving the quality of stroke-car$29,310
Funding body: World Stroke Organization
| Funding body | World Stroke Organization |
|---|---|
| Project Team | Doctor Md Hasnain, Conjoint Associate Professor Carlos Garcia Esperon, Professor Neil Spratt, Conjoint Professor Chris Levi, Doctor Steven Maltby, Professor Rohan Walker, Professor Quazi Mohammad, Professor Badrul Mondal, Professor Mohammad Shah Chowdhury, Professor Md Tauhidul Chowdhury, Dr Shomik Maruf |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2024 |
| Funding Finish | 2025 |
| GNo | G2400794 |
| Type Of Funding | C3500 – International Not-for profit |
| Category | 3500 |
| UON | Y |
20231 grants / $90,529
Development, implementation and evaluation of a virtual reality-based training platform for NIHSS stroke assessment$90,529
Funding body: South Eastern Sydney Local Health District
| Funding body | South Eastern Sydney Local Health District |
|---|---|
| Project Team | Professor Rohan Walker, Doctor Murielle Kluge, Doctor Steven Maltby |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2023 |
| Funding Finish | 2023 |
| GNo | G2300873 |
| Type Of Funding | C2400 – Aust StateTerritoryLocal – Other |
| Category | 2400 |
| UON | Y |
20224 grants / $92,610
Design and Evaluation of Team Based Immersive Simulation Training$33,422
Funding body: Victoria Police
| Funding body | Victoria Police |
|---|---|
| Project Team | Professor Rohan Walker, Doctor Murielle Kluge, Doctor Steven Maltby |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2200945 |
| Type Of Funding | C2300 – Aust StateTerritoryLocal – Own Purpose |
| Category | 2300 |
| UON | Y |
NSW eHealth: Development of Mobile Communications Training Tools$25,500
Funding body: eHealth NSW
| Funding body | eHealth NSW |
|---|---|
| Project Team | Professor Rohan Walker, Doctor Murielle Kluge, Doctor Steven Maltby, Ms Ann Stevenson |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | G2200094 |
| Type Of Funding | C2300 – Aust StateTerritoryLocal – Own Purpose |
| Category | 2300 |
| UON | Y |
Sustainment & Implementation of the TACTICS VR Training Platform – Improving Health Workflow Training to Reduce Clinical Variability$20,000
Funding body: Heart & Stroke Program
| Funding body | Heart & Stroke Program |
|---|---|
| Project Team | S Maltby, FR Walker, N Spratt, C Garcia-Esperon, C Levi, M Kluge |
| Scheme | Program Strategic Investment Grant |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | |
| Type Of Funding | Internal |
| Category | INTE |
| UON | N |
Sustainment & Implementation of the TACTICS VR Training Platform – Improving Health Workflow Training to Reduce Clinical Variability$13,688
Funding body: College Health, Medicine and Wellbeing - The University of Newcastle (Australia)
| Funding body | College Health, Medicine and Wellbeing - The University of Newcastle (Australia) |
|---|---|
| Project Team | S Maltby, FR Walker, M Kluge, N Spratt, C Garcia-Esperon, C Levi |
| Scheme | 2022 Linkage Pilot Research Grant Scheme |
| Role | Lead |
| Funding Start | 2022 |
| Funding Finish | 2022 |
| GNo | |
| Type Of Funding | Internal |
| Category | INTE |
| UON | N |
20211 grants / $254,000
Utilising telemedicine to increase treatment rates and improve clinical outcomes for acute stroke patients$254,000
Funding body: Hunter New England Health
| Funding body | Hunter New England Health |
|---|---|
| Project Team | Prof Neil Spratt, Doctor Carlos Garcia-Esperon, Prof Rohan Walker, Prof Chris Levi, Prof Jennifer Majersik, Ass Prof Andrew Southerland, Dr Chris Oldmeadow, AProf Kenny Lawson, Prof Christine Paul, Rosemary Carney, Dr Steven Maltby |
| Scheme | HMRI Advanced Out of Hospital Care (AOHC) – Telehealth Research Initiative, Part C |
| Role | Investigator |
| Funding Start | 2021 |
| Funding Finish | 2023 |
| GNo | |
| Type Of Funding | Other Public Sector - State |
| Category | 2OPS |
| UON | N |
20202 grants / $192,919
Development, Implementation and Evaluation of State-wide Mixed Reality based training platform for Telestroke$169,000
Funding body: The Agency for Clinical Innovation (ACI) - NSW
| Funding body | The Agency for Clinical Innovation (ACI) - NSW |
|---|---|
| Project Team | Professor Rohan Walker, Doctor Steven Maltby, Doctor Rebecca Hood, Doctor Murielle Kluge |
| Scheme | Research Grant |
| Role | Investigator |
| Funding Start | 2020 |
| Funding Finish | 2021 |
| GNo | G2000809 |
| Type Of Funding | C2300 – Aust StateTerritoryLocal – Own Purpose |
| Category | 2300 |
| UON | Y |
Cultural Burning Virtual Reality-Based Training Application$23,919
Funding body: NSW Department of Local Land Services - Hunter
| Funding body | NSW Department of Local Land Services - Hunter |
|---|---|
| Project Team | Doctor Steven Maltby, Professor Rohan Walker, Doctor Murielle Kluge |
| Scheme | Research Grant |
| Role | Lead |
| Funding Start | 2020 |
| Funding Finish | 2021 |
| GNo | G2000744 |
| Type Of Funding | C2400 – Aust StateTerritoryLocal – Other |
| Category | 2400 |
| UON | Y |
20161 grants / $22,500
Exploring Novel Therapies for Cystic Fibrosis$22,500
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Doctor Hock Tay, Professor Paul Foster, Mr Max Plank, Doctor Steven Maltby |
| Scheme | Project Grant |
| Role | Investigator |
| Funding Start | 2016 |
| Funding Finish | 2016 |
| GNo | G1600577 |
| Type Of Funding | Grant - Aust Non Government |
| Category | 3AFG |
| UON | Y |
20151 grants / $1,500
Keystone Symposium: Hematopoiesis, Colorado USA, 22-27 February 2015$1,500
Funding body: University of Newcastle - Faculty of Health and Medicine
| Funding body | University of Newcastle - Faculty of Health and Medicine |
|---|---|
| Project Team | Doctor Steven Maltby |
| Scheme | Travel Grant |
| Role | Lead |
| Funding Start | 2015 |
| Funding Finish | 2015 |
| GNo | G1500324 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
20143 grants / $44,216
Miltenyi Biotec GentleMACS Octo Dissociator with Heaters $23,566
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Professor Phil Hansbro, Professor Paul Foster, Professor Darryl Knight, Professor Dirk Van Helden, Professor Joerg Mattes, Professor Jodie Simpson, Professor Lisa Wood, Prof LIZ Milward, Dr NATHAN Bartlett, Professor Simon Keely, Doctor Steven Maltby, Doctor Andrew Jarnicki, Doctor Malcolm Starkey, Associate Professor Adam Collison, Doctor Shaan Gellatly |
| Scheme | Equipment Grant |
| Role | Investigator |
| Funding Start | 2014 |
| Funding Finish | 2014 |
| GNo | G1500861 |
| Type Of Funding | Other Public Sector - Commonwealth |
| Category | 2OPC |
| UON | Y |
Virus Infections Change the Bone Marrow: Effects on Immunity, Bone Development and Inflammatory Disease$20,000
Funding body: Hunter Medical Research Institute
| Funding body | Hunter Medical Research Institute |
|---|---|
| Project Team | Doctor Steven Maltby, Mr Max Plank, Doctor Hock Tay, Professor Paul Foster |
| Scheme | Project Grant |
| Role | Lead |
| Funding Start | 2014 |
| Funding Finish | 2014 |
| GNo | G1401394 |
| Type Of Funding | Grant - Aust Non Government |
| Category | 3AFG |
| UON | Y |
Inaugural Future of Experimental Medicine Conference: Inflammation in Disease and Ageing, Sydney Australia, 16-19 March 2014$650
Funding body: University of Newcastle - Faculty of Health and Medicine
| Funding body | University of Newcastle - Faculty of Health and Medicine |
|---|---|
| Project Team | Doctor Steven Maltby |
| Scheme | Travel Grant |
| Role | Lead |
| Funding Start | 2014 |
| Funding Finish | 2014 |
| GNo | G1400166 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
20132 grants / $21,500
DP73 Digital colour and monochrome camera + cellSens software + Xcite120 fluorescence lamp illuminator$20,000
Funding body: NHMRC (National Health & Medical Research Council)
| Funding body | NHMRC (National Health & Medical Research Council) |
|---|---|
| Project Team | Professor Paul Foster, Doctor Alan Hsu, Professor Phil Hansbro, Professor Joerg Mattes, Associate Professor Katie Baines, Professor Jodie Simpson, Professor Rakesh Kumar, Doctor Nicole Hansbro, Doctor Steven Maltby, Associate Professor Ming Yang, Associate Professor Gerard Kaiko, Professor Jay Horvat, Professor Simon Keely, Doctor Andrew Jarnicki, Doctor Michael Fricker |
| Scheme | Equipment Grant |
| Role | Investigator |
| Funding Start | 2013 |
| Funding Finish | 2013 |
| GNo | G1201186 |
| Type Of Funding | Other Public Sector - Commonwealth |
| Category | 2OPC |
| UON | Y |
43rd Annual Scientific meeting of the Australasian Society for Immunology (ASI), Wellington New Zealand, 2 - 5 December 2013$1,500
Funding body: University of Newcastle - Faculty of Health and Medicine
| Funding body | University of Newcastle - Faculty of Health and Medicine |
|---|---|
| Project Team | Doctor Steven Maltby |
| Scheme | Travel Grant |
| Role | Lead |
| Funding Start | 2013 |
| Funding Finish | 2014 |
| GNo | G1300439 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
20123 grants / $518,684
2011 Research Fellowship - DVCR Strategic Appointment (PRCARD)$348,315
Funding body: University of Newcastle
| Funding body | University of Newcastle |
|---|---|
| Project Team | Doctor Steven Maltby |
| Scheme | Research Fellowship |
| Role | Lead |
| Funding Start | 2012 |
| Funding Finish | 2015 |
| GNo | G1100534 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
CIHR Post-Doctoral Research Fellowship$150,000
Funding body: Canadian Institutes of Health Research (CIHR)
| Funding body | Canadian Institutes of Health Research (CIHR) |
|---|---|
| Scheme | Health Research |
| Role | Lead |
| Funding Start | 2012 |
| Funding Finish | 2015 |
| GNo | |
| Type Of Funding | International - Competitive |
| Category | 3IFA |
| UON | N |
Fellowship Start-up Grant$20,369
Funding body: University of Newcastle
| Funding body | University of Newcastle |
|---|---|
| Project Team | Doctor Steven Maltby |
| Scheme | Fellowship Grant |
| Role | Lead |
| Funding Start | 2012 |
| Funding Finish | 2012 |
| GNo | G1200114 |
| Type Of Funding | Internal |
| Category | INTE |
| UON | Y |
20051 grants / $94,000
CIHR / Heart & Stroke Transfusion Science Fellowship (Centre for Blood Research)$94,000
Funding body: CIHR / Heart & Stroke Foundation
| Funding body | CIHR / Heart & Stroke Foundation |
|---|---|
| Project Team | Doctor Steven Maltby |
| Scheme | Transfusion Science Fellowship from Centre for Blood Research (UBC) |
| Role | Lead |
| Funding Start | 2005 |
| Funding Finish | 2010 |
| GNo | |
| Type Of Funding | International - Competitive |
| Category | 3IFA |
| UON | N |
Research Supervision
Number of supervisions
Past Supervision
| Year | Level of Study | Research Title | Program | Supervisor Type |
|---|---|---|---|---|
| 2017 | PhD | Modeling of Respiratory Syncytial Virus-induced Exacerbation of Allergic Airways Disease | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2017 | PhD | Understanding the Mechanisms of Bacterial-Induced Exacerbation of Allergic Airways Disease in a Mouse Model | PhD (Immunology & Microbiol), College of Health, Medicine and Wellbeing, The University of Newcastle | Co-Supervisor |
| 2016 | Honours | The Systemic Impacts of Viral Inflammation on the Structural Integrity of Bone and Haematopoiesis | Medical Science, The University of Newcastle - Faculty of Health and Medicine | Co-Supervisor |
News
News • 25 Nov 2024
HMRI honours 2024’s best at Research Excellence Awards
University of Newcastle neurologist, Conjoint Professor Jeannette Lechner-Scott, has been named Researcher of the Year at the 2024 HMRI Research Excellence Awards.
News • 3 Oct 2023
Virtual reality training to improve emergency stroke nursing care
Emergency department nurses across NSW will gain valuable real-time experience in stroke care thanks to a new virtual reality (VR) training program developed by the Centre for Advanced Training Systems at the University of Newcastle.
Dr Steven Maltby
Position
Casual Academic
School of Biomedical Sciences and Pharmacy
College of Health, Medicine and Wellbeing
Contact Details
| steven.maltby@newcastle.edu.au | |
| Phone | (02) 404 20173 |
| Links |
Research Networks Research Networks Research Networks Research Networks |
Office
| Room | MSB 317 |
|---|---|
| Building | MS Building |
| Location | NEWCASTLE , |